Default
DPH Vaccine Ordering Guidance
Current COVID-19 Vaccine Ordering Phase 1A. Expanded phase begins January 11, 2021. This phase includes healthcare workers (paid or unpaid), laboratory, environmental services, LTCF staff and residents, individuals 65 and older, law enforcement officers, firefighters, and first responders.
https://www.gpha.org/wp-content/uploads/Vaccine-Ordering-Guidance-1-7-21.pdf
COVID-19 Vaccination Sites
If you’re looking for a COVID Vaccination Site in your location, be sure to check back often as the COVID Vaccination Site Locations list will be updated frequently. Additional locations statewide will be added when providers are ready to safely administer vaccine, and as vaccine supply allows.
GDPH COVID-19 Provider Update
Starting Monday, January 11, 2021, adults aged 65+ and their caregivers will be eligible for COVID-19 vaccination as a part of phase 1A+. Continue to visit the DPH website to review the Georgia COVID-19 Vaccine Status Dashboard and obtain important vaccine-related information.
Current Vaccine Phase:
Effective Monday, January 11, 2021, the expanded list of individuals eligible for vaccination in Phase 1-A will include:
- Healthcare workers in clinical settings (e.g., nurses, physicians, EMS, laboratory technicians, environmental services)
- Staff and residents of long-term care facilities
- All law enforcement and fire personnel (including volunteer departments), and first responders
- Adults aged 65 and older (and their caregivers as applicable)
Helpful Reminders:
- Providers are not required to hold supply in reserve for 2nd doses. For example, if you received 100 doses and administered 50 doses, continue to administer the remaining 50 doses to new patients who meet the above Phase 1A+ criteria. Providers must place orders for 2nd doses. Refer to the attached Vaccine Ordering Guidance for more information.
- Use this link to complete the survey to let us know if you are willing to be a mass vaccination site. Responses must be received by January 13, 2021 at 5:00pm.
- Providers must establish and enforce accountability measures related to vaccine distribution. Your orders are dependent on available supply allocation for Georgia and your timely and accurate reporting of inventory and doses administered to GRITS. As required by Georgia law and your vaccine provider agreement these immunizations must be reported within 24 hours of administration. Late or forgone reporting puts provider supply at risk and Georgia residents at risk of transmission and exposure to COVID-19, and additional loss of life. If you are not using GRITS or are having difficulty navigating the system, staff are available to assist Monday – Friday, 8 a.m. to 5 p.m. by calling (866) 483 -2958 or emailing dph-gaimmreg@dph.ga.gov.
- Vaccine storage unit temperatures must be monitored regularly and recorded at the start of each workday. Always record minimum/maximum temperature, date, time, name of person checking/recording temperature, actions taken if a temperature excursion occurred. *Temperature records must be kept for a minimum of three years, or longer if required by your jurisdiction.
General Information:
- Interim Considerations: Preparing for the Potential Management of Anaphylaxis at COVID-19 Vaccination Sites – Locations administering COVID-19 vaccines should adhere to CDC guidance for use of COVID-19 vaccines, including screening recipients for contraindications and precautions, having the necessary supplies available to manage anaphylaxis, implementing the recommended post vaccination observation periods, and immediately treating suspected cases of anaphylaxis with intramuscular injection of epinephrine.
- Providers should not contact Pfizer or Moderna to cancel a vaccine request. If a provider is not able to store a vaccine delivery, they should immediately notify the Georgia Immunization Program by calling 404 657-3158 and also notify their local health department. Public health staff will work to locate a facility where the vaccine can be transferred. The facility that initially requested the vaccine should accept the shipment. Do not open the shipping container. The vaccine should be kept in a secure location until it can be picked up and moved to another location
- Vaccine providers with available vaccine should vaccinate members of the community meeting any of the above Phase 1A+ criteria, not just those within their own staff or facility. Due to limited vaccine supply availability, unless they are in one of the above populations, spouses and family members are not eligible for vaccine administration at this time. The Georgia Department of Public Health will notify COVID vaccine providers when to move to the next phase. Moving to additional phases without approval from DPH is a violation of the vaccine provider agreement.
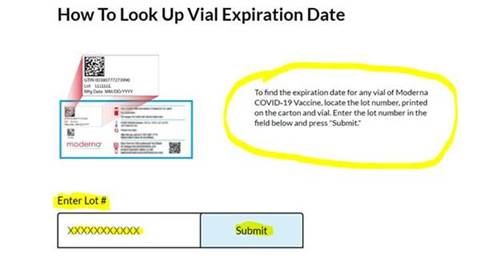
- Visit the Moderna website and scroll to the How To Look Up Vial Expiration Date section to locate vaccine vial expiration date.
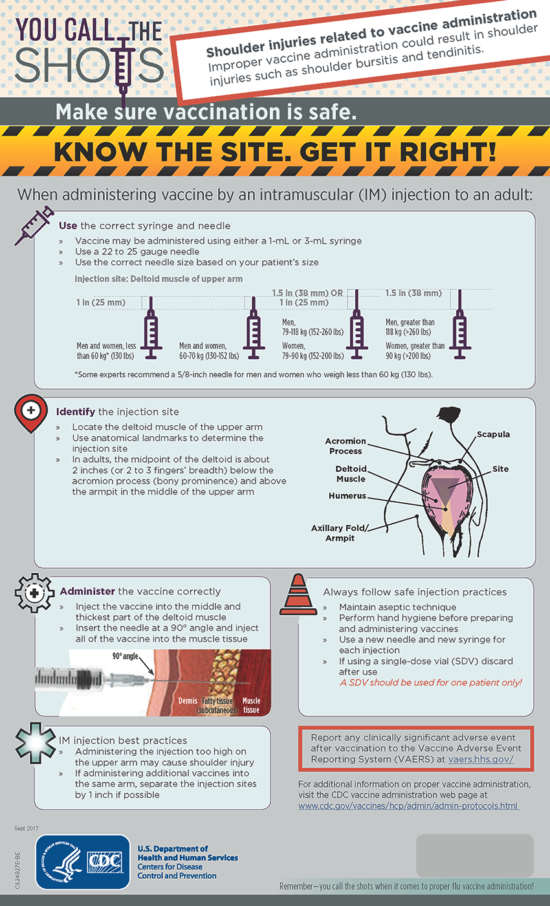
Vaccine Administration Techniques
Preventing Shoulder Injury Related to Vaccine Administration (SIRVA)
All individuals who administer vaccines should reassess their vaccine administration technique and process to ensure use of proper landmark determination and avoidance of patient shoulder injuries related to vaccine administration (SIRVA). There have been increasing numbers of shoulder injury claims associated with vaccine administration by all providers (not just pharmacists). With pharmacists playing increasing roles in vaccine administration, we need to remind practitioners regarding the use of correct vaccine administration technique and procedures.
A Roadmap for Pharmacy Leaders on the COVID-19 Vaccination Journey
 by Phuoc Anne Nguyen PharmD, MS, BCPS and Tracy Nell Dabbs, PharmD, EP Pharmacist
by Phuoc Anne Nguyen PharmD, MS, BCPS and Tracy Nell Dabbs, PharmD, EP Pharmacist
When the COVID-19 vaccines first arrived, there was a lot of excitement and some nervousness among pharmacists. In just 8 short months, we have started to #endCOVID with the initial COVID-19 vaccines and have begun planning to ensure a smooth process. The final goal is to vaccinate as many people as possible. For this article, Tracy and I collaborated to share our two different perspectives.
We want to provide a roadmap for pharmacists leading their COVID-19 vaccination programs. Our hope is to share our lessons learned and strategies to help guide you on your vaccination journey.
Now more than ever, mass immunization will be a healthcare team endeavor to #endCOVID. Close collaboration among interprofessional teams, such as employee health, hospital leadership, supply chain, IT Teams, etc. is the key that will open the door to success! We welcome your comments and invite you to share your lessons learned as well.
We want to provide a roadmap for pharmacists leading their COVID-19 vaccination programs. Our hope is to share our lessons learned and strategies to help guide you on your vaccination journey. READ ARTICLE ON LINKEDIN
Rutledge v PCMA Victory

The Supreme Court of the United States issued its landmark ruling in Rutledge v. Pharmaceutical Care Management Association (PCMA), determining whether community pharmacies are protected from abusive payment practices.
The unanimous (8 to 0) decision ruled in favor of the interests of patients and community pharmacies, who have been fighting for years to regulate pharmacy benefit managers (PBMs), the controversial middlemen that manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. With this ruling, states will have greater authority to protect their local businesses and their patients from PBM overreach.
“This is a historic victory for independent pharmacies and their patients. And it confirms the rights of states to enact reasonable regulations in the name of fair competition and public health,” said National Community Pharmacists Association CEO B. Douglas Hoey, pharmacist, MBA.
“This is a great day for pharmacists and their patients,” said Scott J. Knoer, executive vice president and CEO of the American Pharmacists Association. “For years, PBMs have threatened the sacrosanct relationship between pharmacists and their patients and have never been forced to answer to any authority for their actions. This opinion redresses that imbalance and returns the power to protect the interests of patients to the states and other local authorities, where it belongs.”
“We’re excited to see a unanimous decision from the Court on this case – it’s truly a best case scenario for patients, pharmacists, and pharmacies,” said Rebecca Snead, RPh, NASPA executive vice president and CEO. “Now, it’s time to get to work to make sure states have appropriate PBM regulations in place, and continue to work with our members of Congress to do the same for the federal programs.”
“Today, Arkansas pharmacists join their colleagues across the country to celebrate a triumphant victory years in the making,” said Arkansas Pharmacists Association CEO John Vinson. “The Supreme Court’s ruling means that states can finally protect our patients who receive their pharmacy benefits through their employers. This win should increase drug pricing transparency, increase pharmacy access for patients, improve freedom of choice, and improve the healthcare for our citizens both during and after the pandemic.”
At issue was the extent to which the federal Employee Retirement Income Security Act of 1974 (ERISA), which regulates private employee benefit plans, preempts the states from regulating the amount that PBMs pay pharmacies to dispense prescription drugs that are covered by an employer-sponsored health plan.
NCPA will continue to monitor the case and provide updates here. There are other important cases working their way through the courts, and there will be lots more legislative activity in Congress and the states on which NCPA will be leading the fight. We need your help, so please consider making a donation to the NCPA Legislative/Legal Defense Fund.
Resources • Recording of December 15th post-decision overview of the landmark ruling in Rutledge v. Pharmaceutical Care Management Association (PCMA) • NCPA – Rutledge v. PCMA, No. 18-540 (U.S.): One Pager on Unanimous Decision Reversing Eighth Circuit • C-SPAN – Rutledge, Attorney General of Arkansas v. Pharmaceutical Care Management Association Oral Argument • NCPA – Rutledge v. PCMA, No. 18-540 (U.S.): One-Pager on Oral Argument • NCPA’s Oct. 5 Facebook Live previewing the oral arguments in Rutledge v. PCMA • NCPA’s Sept. 29 webinar previewing the oral arguments in Rutledge v. PCMA • Brief of Arkansas Pharmacists Association, National Community Pharmacists Association, American Pharmacists Association, National Alliance Of State Pharmacy Associations, and Fifty-One Other Pharmacist Associations As Amici Curiae Supporting Petitioner • Latest Information on Rutledge v. PCMA • NCPA Analysis of Rutledge v. PCMA • Rutledge v. PCMA: 15 Years in the Making
From the National Association of Community Pharmacists https://ncpa.org/scotus
Rutledge v. PCMA

From National Community Pharmacists Association (NCPA)
On Oct. 6, 2020, the Supreme Court heard oral argument in Rutledge v. PCMA, No. 18-540 (U.S.), which draws back the curtain on some of the abuses committed by pharmacy benefit managers (PBMs)—or prescription-drug middlemen. At issue is whether a federal law, the Employee Retirement Income Security Act of 1974 (ERISA), invalidates an Arkansas law that places reasonable regulations on PBMs. The Pharmacy Care Management Association (PCMA), the lobbying arm of the PBM industry, has argued that ERISA preempts Arkansas’s Act 900, which ensures that PBMs compensate pharmacists fairly for the medications they dispense to patients.
In an 80-minute argument, the Justices pressed PCMA’s counsel to defend its claims of ERISA preemption. Many of the Justices noted that the main effect of Arkansas’s law is simply to regulate what pharmacists are paid for drugs, and Chief Justice Roberts described PBMs as having “byzantine procedures that affect drug prices.”
Both the State of Arkansas and the federal government argued in defense of Act 900. They noted that PCMA’s argument lacked any limiting principle, because countless State laws have some effect on cost—from medical-practice standards to wage laws—and, thus, could be said to affect ERISA plans. The Supreme Court expressly rejected that argument in its prior decision in N.Y. State Conference of Blue Cross & Blue Shield Plans v. Travelers Insurance Co., 514 U.S. 645 (1995).
Following the argument, the Justices will deliberate privately before issuing a written opinion deciding the case. Although there is no set timetable for a ruling, the Justices typically resolve all cases before the end of the Court’s term, which is set to conclude by the end of June 2021.
The Supreme Court case is the culmination of a years’ long effort led by the National Community Pharmacists Association (NCPA) and the Arkansas Pharmacists Association (APA) to hold PBMs accountable for their abusive practices, which have needlessly restricted patient access to life-saving medications and added billions of dollars annually in unnecessary costs that are ultimately borne by hard-working Americans.
NCPA and APA helped secure the passage of the Arkansas law that the Supreme Court is reviewing, and they have provided support to the State throughout the litigation—at the trial court, the intermediate appellate court, and now the Supreme Court. NCPA and APA also helped secure briefs from a wide range of amici curiae (“friends of the Court”)—from the American Medical Association to AARP—defending Arkansas’s law. In addition, NCPA and APA filed an amici curiae brief—on behalf of pharmacists throughout the country—educating the Court on abusive PBM practices.
For all these reasons, it is no surprise that nearly every State—red and blue—has enacted laws regulating PBMs. And it is also why the federal government and nearly every state—from Texas to California—argued in defense of Arkansas’s law in the Supreme Court.
PBMs are middlemen who have secretly put their own interests—and record-breaking profits—above the patients and plans that they are supposed to serve. State laws like Arkansas’s represent an important step in policing PBMs’ abusive behavior and protecting patient access to affordable medications.
Georgia Pharmacy Foundation Names Liza Chapman as Chairman
NEWS RELEASE
ATLANTA (November 2, 2020)
 The Georgia Pharmacy Foundation is happy to announce Dr. Liza Chapman as chairman, effective immediately. Liza is currently Vice President of Partnership Development for the Pharmacy Technician Certification Board, better known in the industry as PTCB. Prior to joining PTCB, Liza was the pharmacy clinical sales coordinator and residency site coordinator with the Kroger Company in Atlanta, Georgia, where she was employed for over 15 years.
The Georgia Pharmacy Foundation is happy to announce Dr. Liza Chapman as chairman, effective immediately. Liza is currently Vice President of Partnership Development for the Pharmacy Technician Certification Board, better known in the industry as PTCB. Prior to joining PTCB, Liza was the pharmacy clinical sales coordinator and residency site coordinator with the Kroger Company in Atlanta, Georgia, where she was employed for over 15 years.
Liza is a graduate of Mercer University College of Pharmacy and has been licensed since 2002. She has earned APhA certifications in diabetes, dyslipidemia, immunization delivery, and MTM, and is a certified trainer for APhA’s diabetes immunization, and MTM certificate programs. Her track record as a researcher, guest speaker, and CPE instructor is extensive, and she has been published in the Journal of the American Pharmacists Association and has authored chapters in Introduction to the Pharmacy Profession and Community and Clinical Pharmacy Services: A Step-by-Step Approach. In addition, she has been widely recognized as a pharmacy and community leader and has been awarded a number of honors for her work here in Georgia.
When Georgia first considered authorizing pharmacists to administer influenza vaccines under a protocol agreement with physicians, Dr. Chapman took the leading role as GPhA’s spokesperson and volunteer advocate for passage of that law. Her work helped pave the way for expanding the range of immunizations pharmacists can administer in Georgia without a prescription in 2015.
For all of the above and more, Liza was recognized and became an APhA Fellow in 2017. Liza has served in various capacities with GPhA including GPhA President. By now, you should be aware that Liza is an extraordinary leader, person and role model for the pharmacy profession. Welcome back, Liza!
“We are deeply saddened by the passing of our dear colleague and friend, Dr. Jim Bartling, who served the Georgia Pharmacy Foundation for many years. I can never fill Jim’s shoes, but I am honored to have the opportunity to serve again on the Foundation board and continue the legacy that Jim has created.”
-Liza Chapman, PharmD, FAPhA
###
The Georgia Pharmacy Association represents Georgia’s pharmacists, pharmacy technicians, and their patients. We fight for pharmacists at the capitol. We provide education, networking, and resources to improve pharmacy practice — and patients’ lives — every day. For more information and the latest pharmacy news, visit GPhA.org.
The Georgia Pharmacy Foundation is the nonprofit arm of the Georgia Pharmacy Association, which represents the state’s pharmacists, pharmacy technicians, and their patients. The foundation’s mission is to advance the quality of healthcare for patients in Georgia communities through the profession of pharmacy.
Media Contact:
Michelle Turkington
Georgia Pharmacy Association
Director of Marketing and Communications
6065 Barfield Road NE, Suite 100
Sandy Springs, Georgia 30328
mturkington@gpha.org
678-640-4313
Tell us what you want! CPE that is
You can help the GPhA CPE advisory committee deliver top-quality education content for 2021. Please take a few minutes to complete our annual needs assessment to tell us what topics interest you. CLICK HERE to get started!
GPhA Mourns the Passing of Jim Bartling
Georgia lost a pillar of the pharmacy community. It is with the deepest regret that we inform you of the passing of GPhA past President Jim Bartling, PharmD, RPh, GCADC-III, on September 24, 2020.
Jim served as president of the Georgia Pharmacy Association for 1992-93 and served on the Board of Directors of the Georgia Pharmacy Foundation — chairing the foundation until his passing. Jim also co-owned the Medical Arts Pharmacy in Conyers and worked occasionally as a relief pharmacist in the community. In 2009, he was awarded the Bowl of Hygeia, one of the most prestigious recognitions of pharmacist leaders in the state.
Jim’s passion was supporting the recovery of pharmacists, student pharmacists, and pharmacy technicians with substance use disorders. A certified addiction counselor, he was instrumental in the formation of the PharmAssist Program, one of the nation’s first state pharmacy recovery networks (PRNs) – and served as the program’s statewide intervention coordinator. For over 20 years, Dr. Bartling has headed up a private addiction counseling practice that provides outpatient treatment and aftercare monitoring for fellow pharmacists, student pharmacists, and pharmacy technicians.
Jim Bartling spent most of his professional career on the faculty of Mercer University College of Pharmacy, where he earlier earned both his bachelor of science and PharmD. He practiced at Doctors Memorial Hospital in Atlanta before joining what was then the Mercer University Southern School of Pharmacy as director of admissions, job placement, and continuing education; he was later named associate dean for student affairs and admissions, the position from which he retired in 2016. His teaching load included coordinating two elective courses – Drug Misadventures and Substance Abuse.
And even after his retirement, he remained a familiar face on the Mercer campus. Jim’s engagement in pharmacy, in healthcare, and in his community are far too numerous to list. His impact and legacy on the profession and on the Georgia Pharmacy Association and the Georgia Pharmacy Foundation will be felt forever.
You are invited to share your thoughts below with the other Georgia Pharmacy Association members.
U.S. Dept. of Health and Human Services (HHS) authorizes pharmacists to administer all ACIP recommended vaccines to children between the ages of 3 and 18 during the COVID emergency.
Today, August 18, HHS amended its declaration under the Public Readiness and Emergency Preparedness Act to authorize:
“State-licensed pharmacists to order and administer, and pharmacy interns (who are licensed or registered by their state board of pharmacy and acting under the supervision of a state-licensed pharmacist) to administer, any vaccine that the Advisory Committee on Immunization Practices (ACIP) recommends to persons ages three through 18 according to ACIP’s standard immunization schedule (ACIP-recommended vaccines).”
You can read the amendment here: https://www.hhs.gov/sites/default/files/third-amendment-declaration.pdf
This is exciting news as the federal government is recognizing the value of pharmacists and acting on it and GPhA applauds Secretary Azar for this action and is continuing to advocate for emergency action on the state level, particularly with regard to ACIP recommended vaccines as well as forthcoming COVID vaccines for children and adults. Below are the highlights of today’s amended declaration.
Preemption
- Preemption of state law for children between 3 and 18: Georgia law, which does not currently allow pharmacists to order and administer vaccines to children 3 through 12 without a prescription and 13 through 18 only in limited circumstances, is preempted under this declaration as to children between 3 and 18. As such there are no vaccine protocol requirements for children between 3 and 18 and pharmacists are authorized to administer any vaccine that ACIP recommends.
- Vaccine protocol requirements required for 18 and above remain in effect: The declaration does not preempt Georgia law with regard to administering a vaccine without a prescription to those older than 18. Remember, under Georgia law, pharmacists can administer vaccines without a specific prescription for influenza, pneumococcal disease, shingles, or meningitis pursuant to a vaccine protocol agreement and other requirements set forth in O.C.G.A. 43-34-26.1.
Requirements
The authorization applies to Georgia licensed pharmacists and their interns acting under their supervision (intern must be licensed or registered by the Georgia Board of Pharmacy) pursuant to the following requirements:
- The vaccine must be FDA-authorized or FDA-licensed.
- The vaccination must be ordered and administered according to ACIP’s standard immunization schedule.
- The licensed pharmacist must complete a practical training program of at least 20 hours that is approved by the Accreditation Council for Pharmacy Education (ACPE). This training program must include hands-on injection technique, clinical evaluation of indications and contraindications of vaccines, and the recognition and treatment of emergency reactions to vaccines.
- The licensed or registered pharmacy intern must complete a practical training program that is approved by the ACPE. This training program must include hands-on injection technique, clinical evaluation of indications and contraindications of vaccines, and the recognition and treatment of emergency reactions to vaccines.
- The licensed pharmacist and licensed or registered pharmacy intern must have a current certificate in basic cardiopulmonary resuscitation.
- The licensed pharmacist must complete a minimum of two hours of ACPE-approved, immunization-related continuing pharmacy education during each state licensing period.
- The licensed pharmacist must comply with recordkeeping and reporting requirements of the jurisdiction in which he or she administers vaccines, including informing the patient’s primary-care provider when available, submitting the required immunization information to the State or local immunization information system (vaccine registry), complying with requirements with respect to reporting adverse events, and complying with requirements whereby the person administering a vaccine must review the vaccine registry or other vaccination records prior to administering a vaccine.
- The licensed pharmacist must inform his or her childhood-vaccination patients and the adult caregivers accompanying the children of the importance of a well-child visit with a pediatrician or other licensed primary-care provider and refer patients as appropriate.
Note 1: Some of the above requirements are consistent with those under Georgia law and some are more stringent. By way of example in Georgia pharmacists have to report to GRITS but don’t have to check prior to administering. The above is more stringent, providing pharmacists must “review vaccine registry or other vaccination records prior to administering a vaccine.” In other words, don’t assume because you have met the requirements for a vaccine protocol agreement in Georgia you are good to go.
Note: 2: With regard to record-keeping and reporting, the authorization references complying with “record-keeping and reporting requirements of the jurisdiction.” Pharmacists would do well to comply with record-keeping and reporting requirements set forth in O.C.G.A. 43-34-26.1 in addition to any more stringent requirements set forth above.
AIP Spring Meeting cancelled
For obvious reasons, GPhA’s Academy of Independent Pharmacists has cancelled its scheduled Spring Meeting, which was going to be held April 19. If the meeting is going to be rescheduled, we’ll be in touch with members. For now, stay healthy and help your patients do the same.
Legislative Update: Session suspended
The Georgia General Assembly has suspended its 2020 session in the wake of the coronavirus.
“The House and Senate will be gaveled in Friday morning and then reconvene for the 30th legislative day “at a future date and time to be set” by the lieutenant governor and speaker of the House.”
It’s that time of year
Time to nominate someone to be honored with a 2020 GPhA award. If you know a pharmacist who has demonstrated the kind of professional commitment, dedication, or innovative spirit, let GPhA recognize it!
Click the big button to the right, or go to GPhA.org/awards to fill out the nomination form today!
Happy Thanksgiving!
To all our members across the state of Georgia (and beyond!)
To our volunteers who give of their time throughout the year, the pharmacists and techs who fill our classrooms;
the PharmPAC and foundation supporters, the citizen lobbyists who came to the Gold Dome;
the letter-writers, phone-callers, Facebook commenters, Twitter followers, Buzz readers;
the enthusiastic students and thoughtful mentors;
and to the hundreds of quiet, hard-working members who serve their patients and communities…
We say: Thank you!
◀ Newer Posts Older Posts ▶