COVID-19 vaccines for kids ages 5 to 11
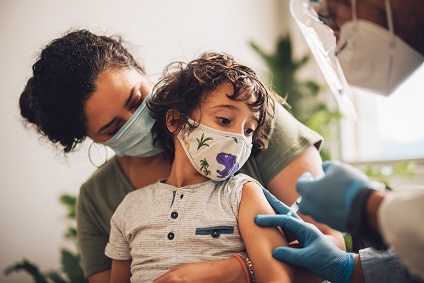 What to expect with COVID-19 vaccines for kids ages 5 to 11
What to expect with COVID-19 vaccines for kids ages 5 to 11
Here’s what the science reveals about the safety of the Pfizer shot for this age group, the doses involved, and the role it will play in protecting everyone from the disease.
Source: National Geographic https://archive.md/B713q#selection-4483.0-4483.174
PUBLISHED OCTOBER 26, 2021
American families who are eagerly awaiting the ability to vaccinate their young children against COVID-19 may finally get their wish in the coming weeks.
Today marks the first step in that process, as a U.S. Food and Drug Administration advisory committee reviews evidence for the safety and effectiveness of the Pfizer-BioNTech shots for kids ages 5 to 11. If the committee approves the immunizations, and if the FDA and the Centers for Disease Control and Prevention follow suit in the coming weeks, the 28 million children in this age group will be able to join their older siblings and parents in getting the jab.
Documents sent to the FDA and released by Pfizer on Friday provide results from their clinical trial with children in this age bracket. The data show that the vaccine offers strong protection for this age group, with a 90.7 percent efficacy rate in preventing symptomatic disease even against the Delta variant now in circulation.
The devastation COVID-19 has wreaked on adults has largely obscured how much children have suffered, says Ofer Levy, Director of the Precision Vaccines Program at Boston’s Children Hospital, who is a member of the FDA committee voting on the Pfizer vaccine today. According to the CDC, nearly 2 million kids 5 to 11 have contracted COVID-19 since the pandemic began, and more than 150 have died.
Had COVID-19 struck as many children as it has without touching a single adult, it would still be a serious public health emergency, Levy says. The question now is how quickly parents will move to vaccinate their young children after shots become available.
When parents were asked last month in a Kaiser Family Foundation survey whether they want their 5 to 11 year old immunized against the coronavirus, 34 percent said they would do it right away. Thirty-two percent want to wait and see, and 7 percent said they will if it’s required, such as by school mandates. Twenty-four percent say they are completely opposed.
“It’s understandable that at this stage parents have a lot of questions,” says Kelly Moore, president and CEO of the nonprofit Immunization Action Coalition, of the group of parents planning to hang back. “People will always be cautious when it comes to their children, and we have not had safety and side effect information for this group before this point,” she says.
Similar dynamics occurred with adults, she notes, but “once people saw how it was working, many were eager to get vaccinated.”
Why kids need vaccines
Given the mass mortality among older adults, it’s easy to lose sight of how children in this younger age group have been impacted by the disease.
In addition to mild or moderate illnesses, more than 5,000 children have developed the serious, full-body reaction to the coronavirus known as Multisystem Inflammatory Syndrome in Children (MIS-C), the vast majority under age 11. The syndrome can cause fever, vomiting, and diarrhea and may lead to heart dysfunction, kidney injury, and, in rare cases, death.
“When you compare COVID’s effects on children to influenza and other diseases that affect them, COVID is much more devastating,” Moore says.
Of course, children suffer even when others develop the disease. Some 140,000 children have lost a primary or secondary caregiver from COVID-19 to date. And the numerous school shut downs and curtailing of social activities have had such a profound psychological effect that the American Academy of Pediatrics and other medical groups have declared children’s mental health to be in a national state of emergency.
What’s more, protecting children with the shots adds to the defenses for all members of their family, especially those younger than 5 who would still not yet be eligible, or any adults at risk of severe disease.
Moore has a friend whose husband is on immunosuppressing drugs to protect his kidney transplant. “Their 8-year-old daughter can’t even go into an ice-cream store because her father is vulnerable if she were to catch and transmit COVID,” she says.
A recent Swedish study confirmed the value of this ring of protection: Families where one member is immunized have up to a 61 percent lower risk that others in the home will get COVID-19, while three or four immunized members gives more than a 90 percent reduction.
Inoculating children in an effort to protect others already happens in the U.S., Levy says. “Some say it’s not ethical to vaccinate kids for a disease that doesn’t affect them as much,” he says, but children are currently immunized against rubella when the main risk is to pregnant mothers, he points out.
A smaller dose
Tens of thousands of adults were tested in Pfizer-BioNTech’s original clinical trials, and with 105,000 Americans over age 12 having completed the two-dose series, the FDA already has extensive information on the effectiveness of the shots. To test the vaccine for children 5 to 11, a different type of trial was conducted, largely focused on safety and dosage.
In the first phase of the trial, Pfizer gave a small group of children either the same 30 microgram dose used for those 12 and older, or they administered 20 or 10 microgram doses. This is a process known as a dose de-escalation trial, says Onyema Ogbuagu, an infectious diseases specialist at Yale Medicine and a principal investigator of the Pfizer trials.
“You want to find the dose that gives a strong immune response while trying to limit adverse events,” he says. The two-dose regimen of 10 micrograms each eventually won out. Pfizer is presenting the test results from some 2,268 participants to the FDA.
The FDA’s Vaccines and Related Biological Products Advisory Committee will comb through every bit of Pfizer’s data before deciding whether to recommend its authorization for 5 to 11s. Pfizer is also currently studying even smaller doses for children between 2 and 5 years old and for those between 6 months and less than 2 years. And more good news for parents of young children: Moderna announced yesterday that its clinical trial in 6 to 11 year olds also produced a robust immune response.
Side effects seen in the Pfizer trial were similar to those for older children, including short-term injection site pain, fatigue, headache, and chills. There were no serious adverse events linked to the vaccine. “You can never say never in medicine, but we feel pretty confident that nothing untoward is expected when even more children get the vaccines,” Ogbuagu says.
Rare events that happen in 1-in-10,000 or 1-in-100,000 people will not emerge until that many children have been vaccinated. The rare cases of the heart inflammation known as myocarditis, which has primarily impacted male adolescents and young men after their mRNA vaccine series, is estimated to occur in roughly 1 in 26,000 males, and nearly all have since recovered.
Addressing parents’ concerns
When weighing any potential risks, parents must compare a vaccine with the disease it aims to protect their child against, Moore says. Even mild cases of COVID-19 can make children feel awful and keep them from attending school. Plus, an unknown number of children continue to suffer for months after their acute illness, a condition that’s come to be known as long COVID.
Among parents who worry, some are concerned about stimulating their child’s immune system with a vaccine, Ogbuagu says, but he counters that “the stimulation it gets when a person gets COVID is much more intense.”
Other resisters focus on the vaccine’s delivery system—the mRNA that instructs the body to create spike proteins for the immune system’s response. But vaccines routinely given to children for other diseases use many different technologies and most parents haven’t much cared, says Robert Jacobson, medical director of the Primary Care Immunization Program at the Mayo Clinic in Rochester, Minnesota.
For example, he says, the measles, mumps, and rubella (MMR) jabs and the chicken pox shots use a weakened form of a live virus. The vaccine for diphtheria employs an altered form of the bacteria. And hepatitis B’s vaccine involves tricking yeast through recombinant technology to produce a protein they don’t naturally make.
What’s more, Ogbuagu says, RNA-based viruses like influenza or respiratory syncytial virus (RSV) routinely infect kids, so it’s not as if their bodies haven’t seen the structure before. “If people knew the host of RNA viruses that enter their child’s cells all the time, they would worry less about the vaccine,” he says.
Pfizer-BioNTech’s shots are already proving valuable for children ages 12 to 17. Some 11 million Americans in this age group, or 57 percent of the total, have gotten at least one shot, while 47 percent are fully vaccinated, according to the AAP. The vaccines have been 93 percent effective in protecting kids this age from going to the hospital, the CDC announced last week.
If the FDA and CDC ultimately authorize the vaccine for kids 5 to 11, Levy wants the shots to be mandated for school, as others currently are. In a recent medical journal editorial, he writes that decreasing virus circulation in children may be our best hope for controlling the spread of COVID-19.
“We’re not out of the woods: We have the winter months coming, and maybe some other variant will emerge. Protecting children and their families could help a lot,” Levi says.
Other doctors simply want to convince families that the shots are a smart approach even if they are not mandated. “As a primary care pediatrician. I will recommend that children get this vaccine as strongly as I do for flu, pertussis, MMR, and others routinely recommended for this age group,” Jacobson says.
“The research shows it to be safe and effective, and with the coronavirus still in widespread circulation, there is clearly a need.”













