Default
Job Posting VP AIP
The Academy of Independent Pharmacy of the Georgia Pharmacy Association seeks a dynamic leader for the position of Vice President of the academy. The position is located in Sandy Springs, Georgia.
The vice president’s position is responsible for growing the AIP Membership through continually increasing the value proposition of academy membership. The position supervises up to five people, so prior supervisory experience is required. For further information about the position, please review the job description.
If interested, please send your resume or CV to asullivan@gpha.org, along with your salary history no later than October 29, 2021. References will be required if your resume is selected for follow up.
Questions about the position may be sent to bcoleman@gpha.org.
View job description.
Scam Alert BOP
UPDATE: SCAM ALERT: “Caller ID Spoofing” of Board of Pharmacy Phone Number
SEPTEMBER 27, 2021
https://gbp.georgia.gov/press-releases/2021-09-27/updatescamalertcalleridspoofing
It has come to our attention that scammers have begun spoofing the telephone number of the Board of Pharmacy (404) 651-8000. If you receive a call and are unsure, please call (404) 651-8000 to verify and report the matter.
Scenario 1: The caller claims to be associated with the Board or Georgia Drugs and Narcotics Agency and tells the pharmacist that his/her license has been revoked or that he/she is under investigation due to a complaint and may not speak to any third party.
Scenario 2: The caller claims to be associated with the Board or Georgia Drugs and Narcotics Agency and tells the Pharmacist that the Board is taking over recalls and requests wholesale account numbers for a “system update.”
Information on Caller ID Spoofing can be found here:
Caller ID Spoofing | Federal Communications Commission (fcc.gov)
CDC Recommends Pfizer Booster
https://www.cdc.gov/coronavirus/2019-ncov/vaccines/booster-shot.html

Now that recommendations for the Pfizer-BioNTech COVID-19 vaccine boosters have been announced by the CDC you may need some clarity on what it means for you and your patients. We’ve compiled the latest information so you can clearly communicate and speak confidently about booster doses in your pharmacy, including who can get a booster dose, how to verify eligibility, and how to document receipt of the vaccine.
Booster Does Eligibility
A single Pfizer-BioNTech COVID-19 vaccine booster dose is now recommended for specific individuals at least 6 months after completing their Pfizer-BioNTech primary series. Pharmacists can begin to administer these doses to eligible individuals.
The following individuals should receive a booster dose:
- Persons aged ≥65 years
- Long-term Care Facility (LTCF) residents
- Persons aged 50-64 years with underlying medical conditions
The following individuals may receive a booster dose:
- Persons aged 18-49 years with underlying medical conditions based on their individual benefits and risk
- Persons aged 18-64 years who are at increased risk for COVID-19 exposure and transmission because of occupational or institutional setting, based on their individual benefits and risks. (This would include healthcare workers, teachers, and other essential workers.)
CDC will be releasing further detailed updates to their Interim Clinical Considerations for Use of COVID-19 Vaccines with more information in the coming days.
|
|
|||||||||||||||||||||||||||||||||||||||
CDC Cautions State Health Officials


More on FDA News Release:
The focus on a Pfizer booster presents real-world challenges for front-line health officials. About 81 million people have received shots made by Moderna and Johnson & Johnson, but their eligibility for a booster was not addressed during the FDA advisory committee meeting and may not be decided for weeks.
On Sunday, Dr. Anthony Fauci, Chief Medical Adviser to the White House COVID-19 response, said data for an extra shot of the Moderna and Johnson & Johnson vaccines is a few weeks away from being presented to the FDA. “We’re working on that right now, to get the data to the FDA so they can examine it and make a determination about the boosters for those people,” Fauci said on NBC’s Meet the Press. “They’re not being left behind by any means.”
The CDC cautioned state health officials last week not to give booster shots until the FDA and CDC take action.
In an email sent to states Thursday, the CDC wrote: “As we have informed you previously, the administration has been making preparations to begin a COVID-19 booster shot rollout as early as the week of September 20. Any implementation of booster programs should begin after FDA takes a regulatory action and ACIP and CDC make clinical recommendations for use.”
FDA Approves Pfizer Booster
News Release
September 22, 2021
The U.S. Food and Drug Administration amended the emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 Vaccine to allow for use of a single booster dose, to be administered at least six months after completion of the primary series in:
- individuals 65 years of age and older;
- individuals 18 through 64 years of age at high risk of severe COVID-19; and
- individuals 18 through 64 years of age whose frequent institutional or occupational exposure to SARS-CoV-2 puts them at high risk of serious complications of COVID-19 including severe COVID-19.
The authorization applies only to the Pfizer-BioNTech COVID-19 Vaccine.
“Today’s action demonstrates that science and the currently available data continue to guide the FDA’s decision-making for COVID-19 vaccines during this pandemic. After considering the totality of the available scientific evidence and the deliberations of our advisory committee of independent, external experts, the FDA amended the EUA for the Pfizer-BioNTech COVID-19 Vaccine to allow for a booster dose in certain populations such as health care workers, teachers and day care staff, grocery workers and those in homeless shelters or prisons, among others,” said Acting FDA Commissioner Janet Woodcock, M.D. “This pandemic is dynamic and evolving, with new data about vaccine safety and effectiveness becoming available every day. As we learn more about the safety and effectiveness of COVID-19 vaccines, including the use of a booster dose, we will continue to evaluate the rapidly changing science and keep the public informed.”
The Process for Assessing the Available Data
Comirnaty (COVID-19 Vaccine, mRNA), was approved by the FDA on Aug. 23, for the prevention of COVID-19 caused by SARS-CoV-2 in individuals 16 years of age and older. On Aug. 25, 2021, the FDA received a supplement from Pfizer Inc. to their biologics license application for Comirnaty seeking approval of a single booster dose to be administered approximately six months after completion of the primary vaccination series for individuals 16 years of age and older.
As part of the FDA’s commitment to transparency, the agency convened a public meeting of its Vaccines and Related Biological Products Advisory Committee (VRBPAC) on Sept. 17 to solicit input from independent scientific and public health experts on the data submitted in the application. During the meeting, the vaccine manufacturer presented information and data in support of its application. The FDA also presented its analysis of clinical trial data submitted by the vaccine manufacturer. Additionally, the public was also given an opportunity to provide comment; and FDA invited international and U.S. agencies and external groups, including representatives from the Israeli Ministry of Health, the University of Bristol, U.K. and the Centers for Disease Control and Prevention, to present recent data on the use of vaccine boosters, epidemiology of COVID-19, and real-world evidence on vaccine effectiveness.
The FDA considered the data that the vaccine manufacturer submitted, information presented at the VRBPAC meeting, and the committee’s discussion, and has determined that based on the totality of the available scientific evidence, a booster dose of Pfizer-BioNTech COVID-19 Vaccine may be effective in preventing COVID-19 and that the known and potential benefits of a booster dose outweigh the known and potential risks in the populations that the FDA is authorizing for use. The booster dose is authorized for administration to these individuals at least six months following completion of their primary series and may be given at any point after that time.
It’s important to note that the FDA-authorized Pfizer-BioNTech COVID-19 Vaccine is the same formulation as the FDA-approved Comirnaty and the vaccines may be used interchangeably.
“We’re grateful for the advice of the doctors, scientists, and leading vaccine experts on our advisory committee and the important role they have played in ensuring transparent discussions about COVID-19 vaccines. We appreciate the robust discussion, including the vote regarding individuals over 65 years of age and individuals at high risk for severe disease, as well as the committee’s views regarding the use of a booster dose for those with institutional or occupational exposure to SARS-CoV-2,” said Peter Marks, M.D., Ph.D., director of FDA’s Center for Biologics Evaluation and Research. “The FDA considered the committee’s input and conducted its own thorough review of the submitted data to reach today’s decision. We will continue to analyze data submitted to the FDA pertaining to the use of booster doses of COVID-19 vaccines and we will make further decisions as appropriate based on the data.”
Data Supporting Authorization for Emergency Use
To support the authorization for emergency use of a single booster dose, the FDA analyzed safety and immune response data from a subset of participants from the original clinical trial of the Pfizer-BioNTech COVID-19 Vaccine. In addition, consideration was given to real-world data on the vaccine’s efficacy over a sustained period of time provided by both U.S. and international sources, including the CDC, the UK and Israel. The immune responses of approximately 200 participants 18 through 55 years of age who received a single booster dose approximately six months after their second dose were assessed. The antibody response against SARS-CoV-2 virus one month after a booster dose of the vaccine compared to the response one month after the two-dose primary series in the same individuals demonstrated a booster response.
Additional analysis conducted by the manufacturer, as requested by the FDA, compared the rates of COVID-19 accrued during the current Delta variant surge among original clinical trial participants who completed the primary two-dose vaccination series early in the clinical trial to those who completed a two-dose series later in the study. The analysis submitted by the company showed that during the study period of July and August 2021, the incidence of COVID-19 was higher among the participants who completed their primary vaccine series earlier, compared to participants who completed it later. The FDA determined that the rate of breakthrough COVID-19 reported during this time period translates to a modest decrease in the efficacy of the vaccine among those vaccinated earlier.
Safety was evaluated in 306 participants 18 through 55 years of age and 12 participants 65 years of age and older who were followed for an average of over two months. The most commonly reported side effects by the clinical trial participants who received the booster dose of the vaccine were pain, redness and swelling at the injection site, as well as fatigue, headache, muscle or joint pain and chills. Of note, swollen lymph nodes in the underarm were observed more frequently following the booster dose than after the primary two-dose series.
Since Dec. 11, 2020, the Pfizer-BioNTech COVID-19 Vaccine has been available under EUA for individuals 16 years of age and older. The authorization was expanded on May 10, 2021 to include those 12 through 15 years of age, and again on Aug. 12, 2021 to include the use of a third dose of a three-dose primary series in certain immunocompromised individuals 12 years of age and older. EUAs can be used by the FDA during public health emergencies to provide access to medical products that may be effective in preventing, diagnosing, or treating a disease, provided that the FDA determines that the known and potential benefits of a product, when used to prevent, diagnose, or treat the disease, outweigh the known and potential risks of the product.
The amendment to the EUA to include a single booster dose was granted to Pfizer Inc.
###
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, products that give off electronic radiation, and for regulating tobacco products.
Judge Rules in Favor of Compounders
Judge rules in favor of compounders, against FDA in lawsuit over Memorandum of Understanding (MOU)
A federal district court judge has issued a summary judgment in favor of a group of compounding pharmacies, ruling that FDA’s MOU on interstate shipments of compounded drugs violated the law, and the agency cannot enforce it.
The MOU will be remanded back to FDA, and the agency must “either certify that it will not have a significant economic effect on small businesses or prepare a regulatory flexibility analysis.”
While obviously not the end of issue, the ruling will allow APC and its coalition partners more time to work with FDA and boards of pharmacy to hopefully craft an MOU that’s less onerous for states to implement and which doesn’t expand FDA oversight beyond what the statute mandates.
The ruling was in response to a suit brought by a group of seven compounding pharmacies, led by Birmingham, Ala.-based Wellness Pharmacy, alleging several significant issues with the process FDA used to develop the MOU. It was a risky move for those pharmacies, but one that paid off.
The plaintiff pharmacies were Medquest Pharmacy, Wellness Pharmacy, Madame Rx, KEBD Enterprises, Hartley Medical Center Pharmacy, Womens International Pharmacy, and VLS Pharmacy. APC was party to an amicus brief filed on behalf of the plaintiffs.
The court found that, because FDA made arbitrary decisions that have “significant binding legal consequences for plaintiffs and pharmacies across the country, and […] signals a substantive change in the current legal regime governing interstate compounding,” the MOU becomes a legislative rule. “As a result,” wrote United States District Judge Christopher Cooper, “FDA was required to comply with the Regulatory Flexibility Act before issuing it. It did not.”
“This was a great piece of news,” said APC Board Chair Shawn Hodges. “We were certain FDA overstepped its authority. And it was heartening to have Judge Cooper see the logic in our argument. I think it will help bring us to an MOU that’s fairer, and hopefully one APC can wholeheartedly endorse.”
Because it was released just this afternoon, we haven’t had time to study the 38-page ruling in depth. We’ll have a more detailed analysis in this week’s Compounding Connections newsletter, and we’ll certainly keep members informed of what happens next. It won’t be long: “The Court,” Cooper wrote, “will request a progress report from the agency within sixty days.”
Jeff Lurey Retirement
NEWS RELEASE
For Immediate Release
GPhA’s Jeff Lurey Retires after an Accomplished Career
 ATLANTA (September 2021), Georgia Pharmacy Association (GPhA) announces the retirement of Jeff Lurey, RPh, GPhA, VP of Independent Pharmacy, effective January 1, 2022. “Jeff has had a long and accomplished pharmacy career. He’s a pharmacy icon,” said Bob Coleman, GPhA CEO.
ATLANTA (September 2021), Georgia Pharmacy Association (GPhA) announces the retirement of Jeff Lurey, RPh, GPhA, VP of Independent Pharmacy, effective January 1, 2022. “Jeff has had a long and accomplished pharmacy career. He’s a pharmacy icon,” said Bob Coleman, GPhA CEO.
Lurey grew up in Warrenton and then Winder, Georgia, graduating from Winder/Barrow High School in 1965. He then attended UGA, studying chemistry, and went on to UGA School of Pharmacy (1965-1970). When asked why he went into pharmacy, Lurey said, “I have always liked science and math. Pharmacy allowed me to combine the two disciplines and make a good living at the same time.” He bought his first pharmacy in 1973, and eventually owned five other apothecary-style pharmacies, which he sold in 1995.
“I think GPhA is the best state pharmacy association in the country (and has been for a very long time),” said Lurey. He has been a member of American Pharmacists Association (APhA) since 1967, and a member of GPhA since I970. He is a member of GPhA, APhA, National Community Pharmacists Association (NCPA), American College of Apothecaries (ACA), and many other social/civic/homeowner associations.
During his accomplished pharmacy career, Lurey served as the president of GPhA’s 5th Region in 1980 and ran for 2nd vice president in 1982. That started a long period of leadership service in numerous positions within GPhA (2nd vice president, 1st vice president, president, and chairman of the board).
Lurey helped start the Georgia Pharmacy Foundation and served as its chairman for many years. He also served on the Georgia Board of Pharmacy (12 years), the UGA Vision Plus Board, and the UGA Alumni Board. He has received the following awards:
- GPhA Appreciation Award
- GPhA Bowl of Hygeia Award
- GPhA 50-year Plaque
- GPhA Mal T. Anderson Outstanding Region President Award
- Mercer University Southern School of Pharmacy Carlton Henderson Award
- Merck Sharpe and Dohme Leadership Award
- McKesson Leadership Award
- NARD (now NCPA) Outstanding Leadership Award
- UGA Vision Plus Award
- UGA Distinguished Alumni Award
When asked to reflect on his 50 years of pharmacy, Lurey told us the biggest change during his tenure was computers. He remembers using manual typewriters to type labels and filing prescriptions by hand, “counting and pouring, licking and sticking.” Patient counseling (Medication Therapy Management (MTM) and Disease State Management (DSM) has also been a major impact on the services that pharmacists provide. Lurey said, “The most significant “negative impact” on pharmacy, in my opinion, has been the total take-over of our profession by Pharmacy Benefit Managers (PBM). I am optimistic we will regain control of our profession and I hope I live long enough to see that happen.”
Lurey is married to his wife Dale (for 51 years). They met at UGA in 1967 and it was love at first sight. They have one son, Alex (born 1974). Alex and his wife Tracy have two children, Richard (13) and Maren (10). They are all big animal lovers and have always had cats and dogs. The newest addition to the family is a cat named Willow. Lurey tells us he will eventually retire to Amelia Island, but will continue to visit Atlanta to see family and watch his grandkids grow up. We asked him what he plans to do in retirement, and he said, “Play a lot of golf.” If you know him, you know that’s right! “We look forward to continuing to work with Jeff in the future in a consultant capacity,” said Coleman. On behalf of the Georgia Pharmacy Association and the pharmacy community, we wish him the best.
###
The Georgia Pharmacy Association represents Georgia’s pharmacists, pharmacy technicians, and their patients. We fight for pharmacists at the capitol. We provide education, networking, and resources to improve pharmacy practice every day. For more information and the latest pharmacy news, visit GPhA.org.
Media Contact:
Holly Hanchey, VP, Marketing & Communications
Georgia Pharmacy Association
hhanchey@gpha.org
404-419-8120
Covid-19 Boosters Aren’t Necessary Yet, Group of Scientists Say
Wall Street Journal article, 9-13-21
Read the WSJ article: https://archive.is/nXLQb
Covid-19 Boosters Aren’t Necessary Yet, Group of Scientists Say.
After data review, scientists say vaccines should go first to people who haven’t gotten them.
An international group of scientists said Monday that there isn’t enough evidence yet to support giving Covid-19 booster shots to the general public.
In a review article published Monday in the Lancet, a group of scientists concluded that current vaccine regimens were still very effective at protecting against severe disease from viral variants, including Delta. They said there doesn’t seem to be a need for boosters to bolster the immunity of the general population yet.
The scientists, including some at the World Health Organization and the U.S. Food and Drug Administration, looked at data from randomized control trials and observational studies published in peer-reviewed journals and preprint servers.
When the scientists averaged the results from observational studies, they said they found that vaccination had 95% effectiveness against severe disease from both the Delta and Alpha variants, and more than 80% effectiveness in protecting against infection from these variants. They noted that waning antibody levels don’t necessarily correlate with a decrease in effectiveness against severe disease. That is because protection against severe disease also comes from other parts of the immune system, they said, such as memory B cells and T-cells.
Boosters may eventually be needed, but there could be risks if boosters were widely introduced too soon or too frequently, the authors of the review said.
“If unnecessary boosting causes significant adverse reactions, there could be implications for vaccine acceptance that go beyond Covid-19 vaccines. Thus, widespread boosting should be undertaken only if there is clear evidence that it is appropriate,” they wrote.
The U.S. has authorized booster shots for certain immunocompromised patients, and the Biden administration is considering plans to broaden distribution to the general public within the month.
Instead of boosters, health authorities should focus on people around the globe who aren’t yet vaccinated, the authors of the Lancet review said. Current vaccine supplies could save more lives if used in unvaccinated populations than if used as boosters in vaccinated populations, they said.
“Even if some gain can ultimately be obtained from boosting, it will not outweigh the benefits of providing initial protection to the unvaccinated,” said Ana-Maria Henao-Restrepo, the study’s lead author and researcher at the WHO. She said vaccines would help inhibit the evolution of further variants and bring the pandemic to an end if they are deployed where fewer people have had access to them so far.
Covid-19 Update Georgia DPH
Tracy Dabbs, PharmD
Aji Kim, (P4) pharmacy student
Covid and Coffee Webinar
September 14, 2021
Georgia Department of Public Health
Covid Update PDF
View webinar here.
Key points with helpful links:
Delta Variant
• Delta (B.1.617.2) is currently the most predominant COVID variant in Georgia
• The Delta variant is more than two times as contagious as other variants
• The greatest risk of COVID transmission is among unvaccinated people
• COVID vaccines are highly effective at preventing severe disease, hospitalization and death
• High vaccination coverage reduces the spread of COVID and helps prevent new variants from emerging
FDA Approval of COVID Vaccines
• FDA approved Pfizer’s COVID vaccine August 23, 2021
• Pfizer vaccine now marketed as Comirnaty (koe-mir’-na-tee)
• The approval is for individuals 16 years of age and older
• Use for Individuals 12 through 15 is authorized by EUA
• 3rd doses for immunocompromised are authorized by EUA
• Moderna has applied for full FDA-approval of its COVID vaccine for individuals aged 18 years and older
• Johnson & Johnson expects to apply for full approval later this year
J&J COVID-19 Vaccine
• J&J COVID-19 vaccine has not been authorized for additional doses or booster doses at this time
• J&J vaccine did not receive EUA until March 2021
• More data is necessary for booster dose information
• Medical experts anticipate those who received J&J would need a booster dose
Pfizer/BioNTech COVID-19 Vaccine Approval
• The FDA-approved Pfizer-BioNTech product COMIRNATY (COVID-19 Vaccine, mRNA) and the FDA-authorized Pfizer-BioNTech COVID-19 Vaccine under EUA have the same formulation and can be used interchangeably to provide the COVID-19 vaccination series without presenting any safety or effectiveness concerns
• Providers can use doses distributed under the EUA to administer the vaccination series for those seeking the approved vaccine.
• The Fact Sheet for Recipients provides additional information about both the approved and authorized vaccine
COVID Vaccine Additional Dose
• CDC recommends an additional dose of vaccine to improve response to the initial vaccine series
• An additional dose of COVID‐19 vaccine would be for individuals who might have not mounted enough immunity due to underlying health conditions, medical treatments, or medications
• Additional doses of Pfizer and Moderna have been authorized for certain immunocompromised patients
• The recommendation currently does not include J&J vaccine recipients
• Individuals receiving active cancer treatment for tumors or cancers of the blood
• Individuals who received an organ transplant and are taking medicine to suppress the immune system
• Individuals who received a stem cell transplant within the last two years or are taking medicine to suppress the immune system
Eligibility for Additional Dose
• Individuals receiving active cancer treatment for tumors or cancers of the blood
• Individuals who received an organ transplant and are taking medicine to suppress the immune system
• Individuals who received a stem cell transplant within the last two years or are taking medicine to suppress the immune system
• Individuals with moderate or severe primary immunodeficiency (such as DiGeorge syndrome, Wiskott-Aldrich syndrome)
• Individuals with advanced or untreated HIV infection
• Individuals with actively being treated with high-dose corticosteroids or other drugs that may suppress the immune response
NOTE: Individuals should talk to their healthcare provider about their medical condition, and whether getting an additional dose is appropriate for them.
COVID Vaccine Booster Dose
• A booster dose of COVID-19 vaccine is for individuals who have completed a vaccination series but have reduced immunity over time
• Pfizer and Moderna have requested permission to administer a third dose of their COVID-19 vaccines (pending FDA authorization and ACIP recommendation)
COVID-19 Vaccine Provider Agreement
• Updated requirements in the CDC COVID-19 Vaccination Program Provider Agreement
• Providers must administer COVID-19 vaccines in accordance with
all program requirements and recommendations of CDC, the Advisory Committee on Immunization Practices, and the U.S Food and Drug Administration (FDA). This applies to both EUA and FDA approved COVID-19 vaccines
• Use of these products outside of those that have been approved and authorized by FDA (often referred to as “off-label use”) is not recommended
Violation of the Vaccine Provider Agreement could expose COVID-19 vaccine providers to the following risks:
• Administration of the product off label may not be covered under the PREP Act or the PREP Act declaration
• Individuals who receive an off‐label dose may not be eligible for compensation under the Countermeasures Injury Compensation Program after a possible adverse event
• CDC has defined the scope of the CDC COVID‐19 Vaccination Program in terms of how the USG‐provided vaccines may be used in the program. Providers giving off‐label doses would be in violation of the CDC Program provider agreement potentially impacting their ability to remain a provider in the CDC program
• Administration fees may not be reimbursable by payers
Vaccine Management System (VMS)
Vaccine Management System is a secure solution for COVID vaccine that enables vaccine management and data sharing for the State of Georgia on one central platform. In VMS, DPH users may:
• View vaccine requests
• View provider information
• View manufacturer and vaccine information
• COVID-19 vaccine providers through DPH will need a VMS account to order COVID-19 vaccine
• Microsoft Office Account requirement
• Questions should be directed to the Provider Support Call Center at: 888‐920‐0165 and DPH-COVID19vaccine@dph.ga.gov
Expanding Access to COVID 19 Therapeutics
HHS PREP Act Declaration: 9th Amendment
• The 9th amendment to the COVID 19 PREP Act Declaration provides liability immunity to and expands the scope of authority for licensed pharmacists to order and administer select COVID 19 therapeutics to populations authorized by the FDA and for pharmacy technicians and pharmacy interns to administer COVID 19 therapeutics
• An example of a COVID 19 therapeutic would be REGEN-COV, a monoclonal antibody developed by Regeneron
Monoclonal Antibodies
• Man-made proteins that mimic human’s immune system’s ability to fight off harmful antigens such as viruses
• Antibody circulate throughout the body until they find their specific antigen and attaches to it
• Once attached to the antigen, other parts of immune system comes along and destroy cells that contain the antigen
Monoclonal Antibody Treatment
- The first monoclonal antibody approved by the FDA was the Orthoclone OKT3 (muromonab-CD3) in 1986.
- Indication: steroid-resistant acute allograft rejection in cardiac and hepatic transplant
- Before the –mab: source
•-o-mab: mouse
• -xi-mab: chimeric (human + non-human)
• -zi-mab: humanized (mostly human + part non-human)
• -u-mab: human - Before the source: targets
• C(i): circulatory system
• K(i): interleukin
• L(i): immune system
• T(u): tumor
• V(i): virus
REGEN-COV (casirivimab + imdevimab)
https://www.phe.gov/emergency/events/COVID19/investigation-MCM/cas_imd.aspx
• Authorized under EUA
• Indication: COVID-19 post exposure prophylaxis (PEP), COVID-19 mild to moderate treatment
• Administration:
• IV (preferred)
• Subcutaneous
HHS is managing the distribution of REGEN-COV
• Interim clinical considerations for use of COVID-19 vaccines currently approved or authorized in the US
• https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
REGEN-COV Formulations
There are TWO different formulations of REGEN-COV
• Casirivimab and imdevimab co-formulated solution containing two antibodies in a 1:1 ratio in a vial
• Casirivimab and imdevimab available as individual antibody solutions in separate vials, supplied in individual vials, and dose pack.
• The dose pack contains individual vials of casirivimab and imdevimab, configurations that may vary in vial size, strength, and appearance
• Side effects: injection site reactions such as skin redness, irritation sensation, and ecchymosis
• Post exposure prophylaxis with REGEN-COV2 is not a substitute for vaccination against COVID-19
• REGEN-COV2 is not authorized for pre-exposure prophylaxis for prevention of COVID-19
• REGEN-COV2 is not authorized for treatment of severe COVID-19 cases due to unavailable data ex: patients with ventilator
Monoclonal Antibody Access in Georgia
- Currently over 150 locations in Georgia where monoclonal antibody treatment is available. https://protect-public.hhs.gov/pages/therapeutics-distribution
- Call Center dedicated to questions and information related to mAbs: English: 877-332-6585 Spanish: 877-366-0310
References
1. https://www.hhs.gov/about/news/2021/08/18/joint-statement-hhs-public-health-and-medical-experts-covid-19-booster-shots.html
2. https://www.cdc.gov/coronavirus/2019-
ncov/vaccines/recommendations/immuno.html
3. https://www.webmd.com/vaccines/covid-19-vaccine/news/20210817/us-to-recommend-covid-vaccine-boosters-8-months
4. https://www.regeneron.com/downloads/treatment-covid19-eua-fact-sheet-for-hcp.pdf
5. https://www.fda.gov/news-events/press-announcements/fda-approves-first-covid-19-vaccine
CDC Phizer-BioNTech Shelf Life Extension
CDC has provided additional guidance related to the Pfizer vaccine expiration date extension as outlined below:
https://www.gpha.org/wp-content/uploads/Pfizer-Extension-Update.pdf
Pfizer-BioNTech Shelf-Life Extension
- FDA has approved the extension of Pfizer expiration dates from six to nine months immediately (no changes to vaccine – based on stability data).
- Eligible Products: Cartons/vials with expiry dates August 2021 – February 2022 printed on the label may remain in use for 3 months beyond the printed date as long as authorized storage conditions between -90°C to -60°C have been maintained (extension also applies to expired product <9 months old that has been kept in ultra-cold storage).
- Not Eligible for the 3-month expiration date extension: Frozen vials stored at -25°C to -15°C; refrigerated vials (however, vials stored at frozen or refrigerated temperatures with a Beyond Use Date (BUD) that extends past 8/31/2021 may be honored. Please do not waste or discard these doses until the BUD has passed).
Updated Expiry Dates – Reference Table
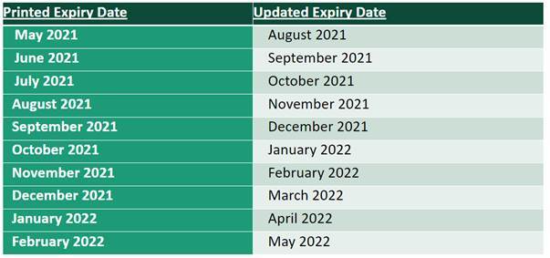
BUD Case Scenarios:
Pfizer-BioNTech COVID-19 Vaccine Expiration Extension
With the Pfizer-BioNTech COVID-19 Vaccine expiration date extension, there have been several questions about how this might impact beyond-use dates. Below are a few case scenarios you might encounter.
To prevent vaccine from inadvertently being discarded, providers should ensure the new expiration date is indicated, i.e., label the tray or post on the unit.
Case scenario #1:
You move a vial of Pfizer vaccine from freezer storage to the refrigerator on August 15. It is marked with the expiration date of August 2021 (August 31, 2021). Can you store this vaccine for the full 31 days allowed for refrigeration storage, or do you need to administer it by August 31, 2021?
-
- Yes, you can store this vaccine vial for the full 31 days (i.e., through September 15, 2021). The recent expiration date extension changes the expiration date to November 2021. The BUD replaces the manufacturer’s expiration date (now November/2021) and should be noted on the label along with the initials of the person making the calculation.
Case scenario #2:
You move a vial of Pfizer vaccine from ultracold storage to the freezer on August 23. It is marked with the expiration date of August 2021 (August 31, 2021). Can you store this vaccine for the full 2 weeks allowed in the freezer? After that time, can you still move it to the refrigerator and store for an additional 31 days?
-
- Yes, you can store this vaccine vial for the full 2 weeks in the freezer (through September 6, 2021), move it to the refrigerator, and store for an additional 31 days (October 7, 2021). The recent expiration date extension changes the expiration date to November 2021. The BUD replaces the manufacturer’s expiration date (now November 2021) and should be noted on the label along with the initials of the person making the calculation.
Case scenario #3:
You move a vial of Pfizer-BioNTech COVID-19 vaccine from the freezer to the refrigerator on August 1, 2021. Since the expiration date has been extended, can you store this vial in the refrigerator through November 31, 2021?
-
- No, you cannot store this vial through the extended expiration date. A vial can be stored in the refrigerator between 2°C and 8°C (36°F and 46°F) for up to 1 month (31 days) regardless of the expiration date extension.
Clinical guidance and resources for storing, preparing and administering the vaccine, including labels to assist with tracking the BUD can be found here: www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/index.html
CDC Health Advisory Ivermectin

Distributed via the CDC Health Alert Network
August 26, 2021, 11:40 AM ET
CDCHAN-00449
Rapid Increase in Ivermectin Prescriptions and Reports of Severe Illness Associated with Use of Products Containing Ivermectin to Prevent or Treat COVID-19
Summary
Ivermectin is a U.S. Food and Drug Administration (FDA)-approved prescription medication used to treat certain infections caused by internal and external parasites. When used as prescribed for approved indications, it is generally safe and well tolerated.
During the COVID-19 pandemic, ivermectin dispensing by retail pharmacies has increased, as has use of veterinary formulations available over the counter but not intended for human use. FDA has cautioned about the potential risks of use for prevention or treatment of COVID-19.
Ivermectin is not authorized or approved by FDA for prevention or treatment of COVID-19. The National Institutes of Health’s (NIH) COVID-19 Treatment Guidelines Panel has also determined that there are currently insufficient data to recommend ivermectin for treatment of COVID-19. ClinicalTrials.gov has listings of ongoing clinical trials that might provide more information about these hypothesized uses in the future.
Adverse effects associated with ivermectin misuse and overdose are increasing, as shown by a rise in calls to poison control centers reporting overdoses and more people experiencing adverse effects.
Background
The Centers for Disease Control and Prevention (CDC) confirmed with the American Association of Poison Control Centers (AAPCC) that human exposures and adverse effects associated with ivermectin reported to poison control centers have increased in 2021 compared to the pre-pandemic baseline. These reports include increased use of veterinary products not meant for human consumption.
Ivermectin is a medication that is approved by FDA in oral formulations to treat onchocerciasis (river blindness) and intestinal strongyloidiasis. Topical formulations are used to treat head lice and rosacea. Ivermectin is also used in veterinary applications to prevent or treat internal and external parasitic infections in animals. When used in appropriate doses for approved indications, ivermectin is generally well tolerated.
Clinical trials and observational studies to evaluate the use of ivermectin to prevent and treat COVID-19 in humans have yielded insufficient evidence for the NIH COVID-19 Treatment Guidelines Panel to recommend its use. Data from adequately sized, well-designed, and well-conducted clinical trials are needed to provide more specific, evidence-based guidance on the role of ivermectin in the treatment of COVID-19.
A recent study examining trends in ivermectin dispensing from outpatient retail pharmacies in the United States during the COVID-19 pandemic showed an increase from an average of 3,600 prescriptions per week at the pre-pandemic baseline (March 16, 2019–March 13, 2020) to a peak of 39,000 prescriptions in the week ending on January 8, 2021.1 Since early July 2021, outpatient ivermectin dispensing has again begun to rapidly increase, reaching more than 88,000 prescriptions in the week ending August 13, 2021. This represents a 24-fold increase from the pre-pandemic baseline. (Figure)
Figure: Estimated number of outpatient ivermectin prescriptions dispensed from retail pharmacies — United States, March 16, 2019–August 13, 2021*
*Data are from the IQVIA National Prescription Audit Weekly (NPA Weekly) database. NPA Weekly collects data from a sample of approximately 48,900 U.S. retail pharmacies, representing 92% of all retail prescription activity. Ivermectin dispensed by mail order and long-term care pharmacies, prescriptions by veterinarians, and non-oral formulations were not included.

In 2021, poison control centers across the U.S. received a three-fold increase in the number of calls for human exposures to ivermectin in January 2021 compared to the pre-pandemic baseline.
In July 2021, ivermectin calls have continued to sharply increase, to a five-fold increase from baseline. These reports are also associated with increased frequency of adverse effects and emergency department/hospital visits.
In some cases, people have ingested ivermectin-containing products purchased without a prescription, including topical formulations and veterinary products. Veterinary formulations intended for use in large animals such as horses, sheep, and cattle (e.g., “sheep drench,” injection formulations, and “pour-on” products for cattle) can be highly concentrated and result in overdoses when used by humans. Animal products may also contain inactive ingredients that have not been evaluated for use in humans. People who take inappropriately high doses of ivermectin above FDA-recommended dosing may experience toxic effects.
Clinical effects of ivermectin overdose include gastrointestinal symptoms such as nausea, vomiting, and diarrhea. Overdoses are associated with hypotension and neurologic effects such as decreased consciousness, confusion, hallucinations, seizures, coma, and death. Ivermectin may potentiate the effects of other drugs that cause central nervous system depression such as benzodiazepines and barbiturates.
Examples of recent significant adverse effects reported to U.S. poison control centers include the following:
- An adult drank an injectable ivermectin formulation intended for use in cattle in an attempt to prevent COVID-19 infection. This patient presented to a hospital with confusion, drowsiness, visual hallucinations, tachypnea, and tremors. The patient recovered after being hospitalized for nine days.
- An adult patient presented with altered mental status after taking ivermectin tablets of unknown strength purchased on the internet. The patient reportedly took five tablets a day for five days to treat COVID-19. The patient was disoriented and had difficulty answering questions and following commands. Symptoms improved with discontinuation of ivermectin after hospital admission.
Recommendations for Clinicians and Public Health Practitioners
- Be aware that ivermectin is not currently authorized or approved by FDA for treatment of COVID-19. NIH has also determined that there are currently insufficient data to recommend ivermectin for treatment of COVID-19.
- Educate patients about the risks of using ivermectin without a prescription, or ingesting ivermectin formulations that are meant for external use or ivermectin-containing products formulated for veterinary use.
- Advise patients to immediately seek medical treatment if they have taken any ivermectin or ivermectin-containing products and are experiencing symptoms. Signs and symptoms of ivermectin toxicity include gastrointestinal effects (nausea, vomiting, abdominal pain, and diarrhea), headache, blurred vision, dizziness, tachycardia, hypotension, visual hallucinations, altered mental status, confusion, loss of coordination and balance, central nervous system depression, and seizures. Ivermectin may increase sedative effects of other medications such as benzodiazepines and barbiturates. Call the poison control center hotline (1-800-222-1222) for medical management advice.
- Educate patients and the public to get vaccinated against COVID-19. COVID-19 vaccination is safe and the most effective means to prevent infection and protect against severe disease and death from SARS-CoV-2, the virus that causes COVID-19, including the Delta variant.
- Educate patients and the public to use COVID-19 prevention measures including wearing masks in indoor public places, physical distancing by staying at least six feet from other people who don’t live in the same household, avoiding crowds and poorly ventilated spaces, and frequent handwashing and use of hand sanitizer that contains at least 60 percent alcohol.
Recommendations for the Public
- Be aware that currently, ivermectin has not been proven as a way to prevent or treat COVID-19.
- Do not swallow ivermectin products that should be used on skin (e.g., lotions and creams) or are not meant for human use, such as veterinary ivermectin products.
- Seek immediate medical attention or call the poison control center hotline (1-800-222-1222) for advice if you have taken ivermectin or a product that contains ivermectin and are having symptoms. Signs and symptoms include gastrointestinal effects (nausea, vomiting, abdominal pain, and diarrhea), headache, blurred vision, dizziness, fast heart rate, and low blood pressure. Other severe nervous system effects have been reported, including tremors, seizures, hallucinations, confusion, loss of coordination and balance, decreased alertness, and coma.
- Get vaccinated against COVID-19. COVID-19 vaccination is approved by FDA and is the safest and most effective way to prevent getting sick and protect against severe disease and death from SARS-CoV-2, the virus that causes COVID-19, including the Delta variant.
- Protect yourself and others from getting sick with COVID-19. In addition to vaccination, wear masks in indoor public places, practice staying at least six feet from other people who don’t live in your household, avoid crowds and poorly ventilated spaces, and wash your hands often or use hand sanitizer that has at least 60 percent alcohol.
For More Information
NIH COVID-19 Treatment Ivermectin Guidelines
FDA Consumer Alert on Use of Ivermectin to Treat or Prevent COVID-19
FDA MedWatch Adverse Event Reporting program
CDC Coronavirus (COVID-19) website
U.S. Government Coronavirus (COVID-19) website
American Association of Poison Control Centers
Treatments Your Healthcare Provider Might Recommend if You Are Sick
References
1 Lind JN, Lovegrove MC, Geller AI, Uyeki TM, Datta SD, Budnitz DS. Increase in Outpatient Ivermectin Dispensing in the US During the COVID-19 Pandemic: A Cross-Sectional Analysis. J Gen Intern Med. 2021 Jun 18:1–3. doi: 10.1007/s11606-021-06948-6.
The Centers for Disease Control and Prevention (CDC) protects people’s health and safety by preventing and controlling diseases and injuries; enhances health decisions by providing credible information on critical health issues; and promotes healthy living through strong partnerships with local, national, and international organizations.
CMS Expands Medicare Payments for At-Home COVID-19 Vaccinations
 FOR IMMEDIATE RELEASE, August 24, 2021
FOR IMMEDIATE RELEASE, August 24, 2021
Contact: CMS Media Relations, (202) 690-6145 | CMS Media Inquiries
Part of Biden-Harris Administration Vaccine Outreach, CMS Boosts Vaccine Access in Smaller Group Homes, Assisted Living Facilities, and Other Group Living Situations
As part of the Biden-Harris Administration’s ongoing commitment to increasing access to vaccinations and improving health equity, the Centers for Medicare & Medicaid Services (CMS) is expanding opportunities for people to receive COVID-19 vaccinations in their home. To ensure Medicare beneficiaries who have difficulty leaving their homes or are otherwise hard-to reach can receive the vaccination, healthcare providers can now receive additional payments for administering vaccines to multiple residents in one home setting or communal setting of a home.
Today’s announcement aims to further boost the administration of COVID-19 vaccination – including second and third doses – in smaller group homes, assisted living facilities, and other group living situations by allowing vaccine providers to receive the increased payment up to five times when fewer than ten Medicare beneficiaries get the vaccine on the same day in the same home or communal setting. This policy will help ensure that at-risk patients in smaller settings have the same opportunities as others to receive the vaccination.
“We are doing everything we can to remove barriers to vaccinations, including ensuring appropriate payment levels for vaccine providers to connect with more people in their communities who are unable to receive the vaccine in a traditional setting,” said CMS Administrator Chiquita Brooks-LaSure. “We’ve seen the difference that vaccinations have in communities, and we are calling on providers to join us as we continue to increase vaccination rates across the country. Today’s actions ensure that everyone has the ability to be vaccinated against COVID-19, including older adults with mobility or transportation challenges and other at-risk individuals.”
While many Medicare beneficiaries are able to receive a COVID-19 vaccine at a retail pharmacy or from a health care provider, some people have great difficulty leaving their homes or cannot easily access vaccination in these settings. These individuals are often at-risk patients who could require complex care if they contracted COVID-19 and needed to be hospitalized. To better serve this group, Medicare previously increased the total payment amount for at-home vaccination from approximately $40 to approximately $75 per vaccine dose, in certain circumstances.
Delivering COVID-19 vaccination to access-challenged and hard-to-reach individuals poses some unique challenges, such as ensuring appropriate vaccine storage temperatures, handling, and administration. Along with the Centers for Disease Control and Prevention’s (CDC) guidance, today’s announcement helps vaccine providers meet these challenges and successfully administer vaccinations.
The additional payment amount also accounts for the clinical time needed to monitor a beneficiary after the vaccine is administered, as well as the upfront costs associated with administering the vaccine safely and appropriately in a beneficiary’s home. The payment rate for administering each dose of a COVID-19 vaccine, as well as the additional in-home payment amount, is geographically adjusted based on where the service is furnished.
How to Find a COVID-19 Vaccine
As states and the federal government continue to break down barriers – like where vaccines can be administered – resources for connecting communities to vaccination options remain key.
Unvaccinated individuals and those looking to assist friends and family can:
- Visit vaccines.gov (English) or vacunas.gov (Spanish) to search for vaccines nearby.
- Text GETVAX (438829) for English or VACUNA (822862) for Spanish for near instant access to details on three vaccine sites in the local area.
- Call the National COVID-19 Vaccination Assistance Hotline at 1-800-232-0233 (TTY: 1-888-720-7489) for assistance in English and Spanish.
Coverage of COVID-19 Vaccines
The federal government is providing the COVID-19 vaccine free of charge or with no cost sharing for Medicare beneficiaries. As a condition of receiving free COVID-19 vaccines from the federal government, vaccine providers cannot charge patients any amount for administering the vaccine.
Because no patient can be billed for COVID-19 vaccinations, CMS and its partners have provided a variety of information online for providers vaccinating all Americans regardless of their insurance status:
- Original Medicare and Medicare Advantage: Beneficiaries with Medicare pay nothing for COVID-19 vaccines or their administration, and there is no applicable copayment, coinsurance or deductible.
- Medicaid and the Children’s Health Insurance Program (CHIP): State Medicaid and CHIP agencies must cover COVID-19 vaccine administration with no cost sharing for nearly all beneficiaries during the COVID-19 public health emergency (PHE) and (generally) for over a year after it ends. For the very limited number of Medicaid beneficiaries who are not eligible for this coverage (and do not receive it through other coverage they might have), providers may submit claims for reimbursement for administering the COVID-19 vaccine to underinsured individuals through the COVID-19 Coverage Assistance Fund, administered by the Health Resources and Services Administration (HRSA), as discussed below.
- Under the American Rescue Plan Act of 2021 (ARP), signed by President Biden on March 11, 2021, the federal matching percentage for state Medicaid and CHIP expenditures on COVID-19 vaccine administration is currently 100% (as of April 1, 2021), and will remain 100% for more than a year after the COVID-19 PHE ends. The ARP also expands coverage of COVID-19 vaccine administration under Medicaid and CHIP to additional eligibility groups. CMS recently updated the Medicaid vaccine toolkit to reflect the enactment of the ARP at https://www.medicaid.gov/state-resource-center/downloads/covid-19-vaccine-toolkit.pdf.
- Private Plans: The vaccine is free for people enrolled in most private health plans. The COVID-19 vaccines and the administration are covered without cost sharing for most enrollees, and such coverage must be provided both in-network and out-of-network during the PHE. Current regulations provide that out-of-network rates must be reasonable as compared to prevailing market rates, and the rules reference using the Medicare payment rates as a potential guideline for insurance companies.
- In light of CMS’s action today, CMS expects health insurance issuers and group health plans to continue to ensure their rates are reasonable when compared to prevailing market rates. Under the conditions of participation in the CDC COVID-19 Vaccination Program, providers cannot charge plan enrollees any administration fee or cost sharing, regardless of whether the COVID-19 vaccine is administered in-network or out-of-network.
For individuals who are underinsured, vaccine providers may submit claims for reimbursement for administering the COVID-19 vaccine through the COVID-19 Coverage Assistance Fund administered by the Health Resources and Services Administration (HRSA) after the claim to the individual’s health plan for payment has been denied or only partially paid. Information is available at https://www.hrsa.gov/covid19-coverage-assistance.
For individuals who are uninsured, vaccine providers may submit claims for reimbursement for administering the COVID-19 vaccine to individuals without insurance through the Provider Relief Fund, administered by the Health Resources and Services Administration (HRSA). Information on the COVID-19 Claims Reimbursement to Health Care Providers and Facilities for Testing, Treatment, and Vaccine Administration for the Uninsured Program is available at https://www.hrsa.gov/CovidUninsuredClaim.
More information on Medicare payment for COVID-19 vaccine administration – including a list of billing codes, payment allowances and effective dates – is available at https://www.cms.gov/medicare/covid-19/medicare-covid-19-vaccine-shot-payment.
More information regarding the CDC COVID-19 Vaccination Program Provider Requirements and how the COVID-19 vaccine is provided through that program at no cost to recipients is available at https://www.cdc.gov/vaccines/covid-19/vaccination-provider-support.html.
###
NCPDP Releases Updated Emergency Preparedness Guidance
NCPDP Releases Updated Emergency Preparedness Guidance on Additional Doses of the COVID-19 Vaccine
The NCPDP Emergency Preparedness Task Group approved a new revision of the NCPDP Emergency Preparedness Guidance v1.10 document for the industry. The new revision addresses how to handle additional doses of the COVID-19 vaccines.
NCPDP recommends:
Additional Doses
An additional dose may be administered to an individual patient based on patient-specific criteria found in the Emergency Use Authorizations (EUA), FDA-approved label or Advisory Committee on Immunization Practices (ACIP) recommendations. According to the CDC, an additional dose is defined as an additional dose of vaccine given to a targeted population from the same formulation of vaccine currently on the market. New formulations of a vaccine would have a different NDC and do not constitute an additional dose under this definition.
When a patient requires an additional dose of a specific Product/Service ID (407-D7), an additional identifier within the claim request may be necessary to specify the additional dose situation. NCPDP recommends the SCC value of 7 “Medically Necessary,” be used in claim requests for additional doses. Payers may need to modify current utilization edits to allow processing of an additional dose of the vaccine. Plans are reminded to communicate any plan-specific claims submission requirements. The use of ICD-10 codes as the sole means of communicating an additional dose is discouraged.
New formulations of the vaccine will have distinct NDCs (i.e., different from the existing) where the Product/Service ID (407-D7) value would identify the product administered as a distinct product. Existing logic for single or multiple doses should be leveraged to apply applicable claim utilization rules. Rejecting claims for therapeutic duplication based on prior claims for a different NDC is discouraged.
For details, refer to the NCPDP EMERGENCY PREPAREDNESS GUIDANCE V1.10 document on the NCPDP.org website.
|
Special Edition – Monday, August 16, 2021 COVID-19 Vaccines Additional Doses: Codes & Payment The FDA amended the emergency use authorizations (EUAs) for both the Pfizer BioNTech COVID-19 vaccine and the Moderna COVID-19 vaccine to allow for an additional dose in certain immunocompromised people. Effective August 12, 2021, CMS will pay to administer additional doses of COVID-19 vaccines consistent with the FDA EUAs, using CPT code 0003A for the Pfizer vaccine and CPT code 0013A for the Moderna vaccine. We’ll pay the same amount to administer this additional dose as we did for other doses of the COVID-19 vaccine (approximately $40 each). We’ll hold and then process all claims with these codes after we complete claims system updates (no later than August 27). Learn more about Medicare COVID-19 vaccine: |
2022 APhA Awards & Grants
2022 APhA Immunization Champion Awards: Call for Nominations – Deadline: September 15, 2021
The APhA Immunization Champion Awards program recognizes individuals and organizations in the pharmacy space who have made extraordinary contributions toward raising vaccination rates. Nominate yourself or your colleagues who have undertaken successful efforts in their communities.
We highly encourage nominations that specifically relate to immunization services during the COVID-19 pandemic. Submit your nomination online by September 15, 2021.
https://apha.secure-platform.com/a/organizations/main/submissions/details/37682
Awards will be presented at APhA2022, March 18–21, 2022.
2022 APhA Call for Applications for APhA Foundation Incentive Grants – Deadline: August 23, 2021
https://www.aphafoundation.org/incentive-grants
Vaccine Confidence Incentive Grants: Pharmacy Residents may apply for a COVID-19 Vaccine
Confidence Incentive Grant ($5000). Preference will be given to proposals which incorporate delivery of vaccinations within individual practices and/or communities, and projects could address one or more of the following focus areas:
- Enhancing COVID-19 vaccine confidence among pharmacists/pharmacy team members, patients and/or communities
- Increasing access to/uptake of COVID-19 vaccines within identified populations: adolescents, underserved or minority populations, rural areas
- Integration of COVID-19 vaccinations with other vaccines, addressing messaging, practice strategies, and/or administration of vaccines
Student pharmacists and pharmacists may apply for an Innovation in Immunization Practices Incentive Grant ($1000). Preference will be given to projects within the following focus areas:
- Implementing and delivering immunization services in a COVID-19 environment; gaining public trust, overcoming vaccine hesitancy
- Optimizing engagement of pharmacy team members in the provision of immunization-related services in a COVID-19 environment – expanding or reinstating routine vaccinations
- Development of referral mechanisms for immunization services within the community, and strengthening the immunization neighborhood
- Advance usage of Immunization Information System (IIS) for immunization assessment and enhanced immunization delivery methods
- Increasing vaccination rates in the adult population, pregnant patients, and college-age students
- Increasing access to and administration of HPV vaccines
- Implementation of new ACIP recommendations in pharmacy practice (ie. pneumococcal)
- Reinstating routine vaccinations
- Childhood/adolescent vaccinations and vaccine services (and engagement of pharmacy with other healthcare professions)
- Addressing access and pharmacy deserts
To apply go to: https://www.aphafoundation.org/incentive-grants
Pharmacy Techs Can Now Administer Flu Shots
Under HHS authorization (PREP Act 10.20.20) trained pharmacy technicians can administer COVID-19 vaccines and ACIP recommended childhood vaccines.
And now, through the 8th amendment (August 4, 2021) issued by HHS Secretary Xavier Becerra, pharmacy techs can also administer seasonal influenza vaccines as recommended by ACIP.
Pharmacy techs must complete ACPE accredited training and operate under the direct supervision of a licensed pharmacist who is also trained according to state guidelines.
GPhA is offering the accredited training! Pharmacy techs will complete 3 hours of home-study and then attend the 3-hour live training on Saturday, October 16, 2021, in Sandy Springs. Space is limited so don’t wait! Click here for all of the details and to register now!
◀ Newer Posts Older Posts ▶















