COVID-19 Vaccination Sites
If you’re looking for a COVID Vaccination Site in your location, be sure to check back often as the COVID Vaccination Site Locations list will be updated frequently. Additional locations statewide will be added when providers are ready to safely administer vaccine, and as vaccine supply allows.
GDPH COVID-19 Provider Update
Starting Monday, January 11, 2021, adults aged 65+ and their caregivers will be eligible for COVID-19 vaccination as a part of phase 1A+. Continue to visit the DPH website to review the Georgia COVID-19 Vaccine Status Dashboard and obtain important vaccine-related information.
Current Vaccine Phase:
Effective Monday, January 11, 2021, the expanded list of individuals eligible for vaccination in Phase 1-A will include:
- Healthcare workers in clinical settings (e.g., nurses, physicians, EMS, laboratory technicians, environmental services)
- Staff and residents of long-term care facilities
- All law enforcement and fire personnel (including volunteer departments), and first responders
- Adults aged 65 and older (and their caregivers as applicable)
Helpful Reminders:
- Providers are not required to hold supply in reserve for 2nd doses. For example, if you received 100 doses and administered 50 doses, continue to administer the remaining 50 doses to new patients who meet the above Phase 1A+ criteria. Providers must place orders for 2nd doses. Refer to the attached Vaccine Ordering Guidance for more information.
- Use this link to complete the survey to let us know if you are willing to be a mass vaccination site. Responses must be received by January 13, 2021 at 5:00pm.
- Providers must establish and enforce accountability measures related to vaccine distribution. Your orders are dependent on available supply allocation for Georgia and your timely and accurate reporting of inventory and doses administered to GRITS. As required by Georgia law and your vaccine provider agreement these immunizations must be reported within 24 hours of administration. Late or forgone reporting puts provider supply at risk and Georgia residents at risk of transmission and exposure to COVID-19, and additional loss of life. If you are not using GRITS or are having difficulty navigating the system, staff are available to assist Monday – Friday, 8 a.m. to 5 p.m. by calling (866) 483 -2958 or emailing dph-gaimmreg@dph.ga.gov.
- Vaccine storage unit temperatures must be monitored regularly and recorded at the start of each workday. Always record minimum/maximum temperature, date, time, name of person checking/recording temperature, actions taken if a temperature excursion occurred. *Temperature records must be kept for a minimum of three years, or longer if required by your jurisdiction.
General Information:
- Interim Considerations: Preparing for the Potential Management of Anaphylaxis at COVID-19 Vaccination Sites – Locations administering COVID-19 vaccines should adhere to CDC guidance for use of COVID-19 vaccines, including screening recipients for contraindications and precautions, having the necessary supplies available to manage anaphylaxis, implementing the recommended post vaccination observation periods, and immediately treating suspected cases of anaphylaxis with intramuscular injection of epinephrine.
- Providers should not contact Pfizer or Moderna to cancel a vaccine request. If a provider is not able to store a vaccine delivery, they should immediately notify the Georgia Immunization Program by calling 404 657-3158 and also notify their local health department. Public health staff will work to locate a facility where the vaccine can be transferred. The facility that initially requested the vaccine should accept the shipment. Do not open the shipping container. The vaccine should be kept in a secure location until it can be picked up and moved to another location
- Vaccine providers with available vaccine should vaccinate members of the community meeting any of the above Phase 1A+ criteria, not just those within their own staff or facility. Due to limited vaccine supply availability, unless they are in one of the above populations, spouses and family members are not eligible for vaccine administration at this time. The Georgia Department of Public Health will notify COVID vaccine providers when to move to the next phase. Moving to additional phases without approval from DPH is a violation of the vaccine provider agreement.
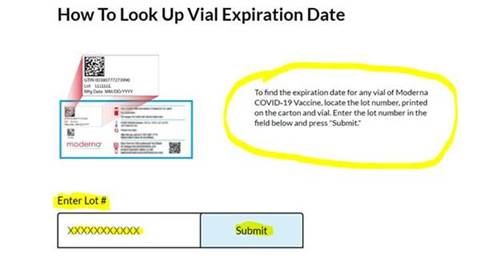
- Visit the Moderna website and scroll to the How To Look Up Vial Expiration Date section to locate vaccine vial expiration date.
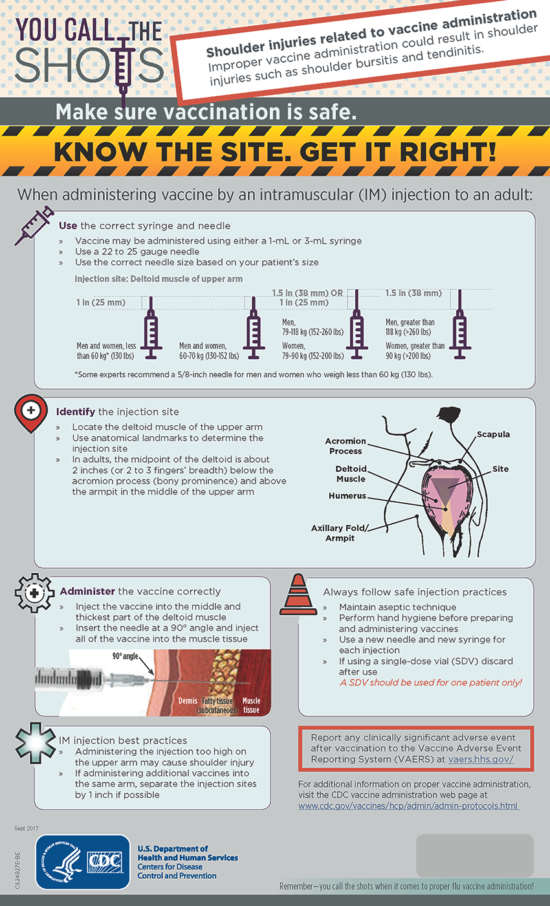
Vaccine Administration Techniques
Preventing Shoulder Injury Related to Vaccine Administration (SIRVA)
All individuals who administer vaccines should reassess their vaccine administration technique and process to ensure use of proper landmark determination and avoidance of patient shoulder injuries related to vaccine administration (SIRVA). There have been increasing numbers of shoulder injury claims associated with vaccine administration by all providers (not just pharmacists). With pharmacists playing increasing roles in vaccine administration, we need to remind practitioners regarding the use of correct vaccine administration technique and procedures.
A Roadmap for Pharmacy Leaders on the COVID-19 Vaccination Journey
 by Phuoc Anne Nguyen PharmD, MS, BCPS and Tracy Nell Dabbs, PharmD, EP Pharmacist
by Phuoc Anne Nguyen PharmD, MS, BCPS and Tracy Nell Dabbs, PharmD, EP Pharmacist
When the COVID-19 vaccines first arrived, there was a lot of excitement and some nervousness among pharmacists. In just 8 short months, we have started to #endCOVID with the initial COVID-19 vaccines and have begun planning to ensure a smooth process. The final goal is to vaccinate as many people as possible. For this article, Tracy and I collaborated to share our two different perspectives.
We want to provide a roadmap for pharmacists leading their COVID-19 vaccination programs. Our hope is to share our lessons learned and strategies to help guide you on your vaccination journey.
Now more than ever, mass immunization will be a healthcare team endeavor to #endCOVID. Close collaboration among interprofessional teams, such as employee health, hospital leadership, supply chain, IT Teams, etc. is the key that will open the door to success! We welcome your comments and invite you to share your lessons learned as well.
We want to provide a roadmap for pharmacists leading their COVID-19 vaccination programs. Our hope is to share our lessons learned and strategies to help guide you on your vaccination journey. READ ARTICLE ON LINKEDIN
Rutledge v PCMA Victory

The Supreme Court of the United States issued its landmark ruling in Rutledge v. Pharmaceutical Care Management Association (PCMA), determining whether community pharmacies are protected from abusive payment practices.
The unanimous (8 to 0) decision ruled in favor of the interests of patients and community pharmacies, who have been fighting for years to regulate pharmacy benefit managers (PBMs), the controversial middlemen that manage prescription drug benefits for health insurers, Medicare Part D drug plans, and large employers. With this ruling, states will have greater authority to protect their local businesses and their patients from PBM overreach.
“This is a historic victory for independent pharmacies and their patients. And it confirms the rights of states to enact reasonable regulations in the name of fair competition and public health,” said National Community Pharmacists Association CEO B. Douglas Hoey, pharmacist, MBA.
“This is a great day for pharmacists and their patients,” said Scott J. Knoer, executive vice president and CEO of the American Pharmacists Association. “For years, PBMs have threatened the sacrosanct relationship between pharmacists and their patients and have never been forced to answer to any authority for their actions. This opinion redresses that imbalance and returns the power to protect the interests of patients to the states and other local authorities, where it belongs.”
“We’re excited to see a unanimous decision from the Court on this case – it’s truly a best case scenario for patients, pharmacists, and pharmacies,” said Rebecca Snead, RPh, NASPA executive vice president and CEO. “Now, it’s time to get to work to make sure states have appropriate PBM regulations in place, and continue to work with our members of Congress to do the same for the federal programs.”
“Today, Arkansas pharmacists join their colleagues across the country to celebrate a triumphant victory years in the making,” said Arkansas Pharmacists Association CEO John Vinson. “The Supreme Court’s ruling means that states can finally protect our patients who receive their pharmacy benefits through their employers. This win should increase drug pricing transparency, increase pharmacy access for patients, improve freedom of choice, and improve the healthcare for our citizens both during and after the pandemic.”
At issue was the extent to which the federal Employee Retirement Income Security Act of 1974 (ERISA), which regulates private employee benefit plans, preempts the states from regulating the amount that PBMs pay pharmacies to dispense prescription drugs that are covered by an employer-sponsored health plan.
NCPA will continue to monitor the case and provide updates here. There are other important cases working their way through the courts, and there will be lots more legislative activity in Congress and the states on which NCPA will be leading the fight. We need your help, so please consider making a donation to the NCPA Legislative/Legal Defense Fund.
Resources • Recording of December 15th post-decision overview of the landmark ruling in Rutledge v. Pharmaceutical Care Management Association (PCMA) • NCPA – Rutledge v. PCMA, No. 18-540 (U.S.): One Pager on Unanimous Decision Reversing Eighth Circuit • C-SPAN – Rutledge, Attorney General of Arkansas v. Pharmaceutical Care Management Association Oral Argument • NCPA – Rutledge v. PCMA, No. 18-540 (U.S.): One-Pager on Oral Argument • NCPA’s Oct. 5 Facebook Live previewing the oral arguments in Rutledge v. PCMA • NCPA’s Sept. 29 webinar previewing the oral arguments in Rutledge v. PCMA • Brief of Arkansas Pharmacists Association, National Community Pharmacists Association, American Pharmacists Association, National Alliance Of State Pharmacy Associations, and Fifty-One Other Pharmacist Associations As Amici Curiae Supporting Petitioner • Latest Information on Rutledge v. PCMA • NCPA Analysis of Rutledge v. PCMA • Rutledge v. PCMA: 15 Years in the Making
From the National Association of Community Pharmacists https://ncpa.org/scotus
Rutledge v. PCMA

From National Community Pharmacists Association (NCPA)
On Oct. 6, 2020, the Supreme Court heard oral argument in Rutledge v. PCMA, No. 18-540 (U.S.), which draws back the curtain on some of the abuses committed by pharmacy benefit managers (PBMs)—or prescription-drug middlemen. At issue is whether a federal law, the Employee Retirement Income Security Act of 1974 (ERISA), invalidates an Arkansas law that places reasonable regulations on PBMs. The Pharmacy Care Management Association (PCMA), the lobbying arm of the PBM industry, has argued that ERISA preempts Arkansas’s Act 900, which ensures that PBMs compensate pharmacists fairly for the medications they dispense to patients.
In an 80-minute argument, the Justices pressed PCMA’s counsel to defend its claims of ERISA preemption. Many of the Justices noted that the main effect of Arkansas’s law is simply to regulate what pharmacists are paid for drugs, and Chief Justice Roberts described PBMs as having “byzantine procedures that affect drug prices.”
Both the State of Arkansas and the federal government argued in defense of Act 900. They noted that PCMA’s argument lacked any limiting principle, because countless State laws have some effect on cost—from medical-practice standards to wage laws—and, thus, could be said to affect ERISA plans. The Supreme Court expressly rejected that argument in its prior decision in N.Y. State Conference of Blue Cross & Blue Shield Plans v. Travelers Insurance Co., 514 U.S. 645 (1995).
Following the argument, the Justices will deliberate privately before issuing a written opinion deciding the case. Although there is no set timetable for a ruling, the Justices typically resolve all cases before the end of the Court’s term, which is set to conclude by the end of June 2021.
The Supreme Court case is the culmination of a years’ long effort led by the National Community Pharmacists Association (NCPA) and the Arkansas Pharmacists Association (APA) to hold PBMs accountable for their abusive practices, which have needlessly restricted patient access to life-saving medications and added billions of dollars annually in unnecessary costs that are ultimately borne by hard-working Americans.
NCPA and APA helped secure the passage of the Arkansas law that the Supreme Court is reviewing, and they have provided support to the State throughout the litigation—at the trial court, the intermediate appellate court, and now the Supreme Court. NCPA and APA also helped secure briefs from a wide range of amici curiae (“friends of the Court”)—from the American Medical Association to AARP—defending Arkansas’s law. In addition, NCPA and APA filed an amici curiae brief—on behalf of pharmacists throughout the country—educating the Court on abusive PBM practices.
For all these reasons, it is no surprise that nearly every State—red and blue—has enacted laws regulating PBMs. And it is also why the federal government and nearly every state—from Texas to California—argued in defense of Arkansas’s law in the Supreme Court.
PBMs are middlemen who have secretly put their own interests—and record-breaking profits—above the patients and plans that they are supposed to serve. State laws like Arkansas’s represent an important step in policing PBMs’ abusive behavior and protecting patient access to affordable medications.
Georgia Pharmacy Foundation Names Liza Chapman as Chairman
NEWS RELEASE
ATLANTA (November 2, 2020)
 The Georgia Pharmacy Foundation is happy to announce Dr. Liza Chapman as chairman, effective immediately. Liza is currently Vice President of Partnership Development for the Pharmacy Technician Certification Board, better known in the industry as PTCB. Prior to joining PTCB, Liza was the pharmacy clinical sales coordinator and residency site coordinator with the Kroger Company in Atlanta, Georgia, where she was employed for over 15 years.
The Georgia Pharmacy Foundation is happy to announce Dr. Liza Chapman as chairman, effective immediately. Liza is currently Vice President of Partnership Development for the Pharmacy Technician Certification Board, better known in the industry as PTCB. Prior to joining PTCB, Liza was the pharmacy clinical sales coordinator and residency site coordinator with the Kroger Company in Atlanta, Georgia, where she was employed for over 15 years.
Liza is a graduate of Mercer University College of Pharmacy and has been licensed since 2002. She has earned APhA certifications in diabetes, dyslipidemia, immunization delivery, and MTM, and is a certified trainer for APhA’s diabetes immunization, and MTM certificate programs. Her track record as a researcher, guest speaker, and CPE instructor is extensive, and she has been published in the Journal of the American Pharmacists Association and has authored chapters in Introduction to the Pharmacy Profession and Community and Clinical Pharmacy Services: A Step-by-Step Approach. In addition, she has been widely recognized as a pharmacy and community leader and has been awarded a number of honors for her work here in Georgia.
When Georgia first considered authorizing pharmacists to administer influenza vaccines under a protocol agreement with physicians, Dr. Chapman took the leading role as GPhA’s spokesperson and volunteer advocate for passage of that law. Her work helped pave the way for expanding the range of immunizations pharmacists can administer in Georgia without a prescription in 2015.
For all of the above and more, Liza was recognized and became an APhA Fellow in 2017. Liza has served in various capacities with GPhA including GPhA President. By now, you should be aware that Liza is an extraordinary leader, person and role model for the pharmacy profession. Welcome back, Liza!
“We are deeply saddened by the passing of our dear colleague and friend, Dr. Jim Bartling, who served the Georgia Pharmacy Foundation for many years. I can never fill Jim’s shoes, but I am honored to have the opportunity to serve again on the Foundation board and continue the legacy that Jim has created.”
-Liza Chapman, PharmD, FAPhA
###
The Georgia Pharmacy Association represents Georgia’s pharmacists, pharmacy technicians, and their patients. We fight for pharmacists at the capitol. We provide education, networking, and resources to improve pharmacy practice — and patients’ lives — every day. For more information and the latest pharmacy news, visit GPhA.org.
The Georgia Pharmacy Foundation is the nonprofit arm of the Georgia Pharmacy Association, which represents the state’s pharmacists, pharmacy technicians, and their patients. The foundation’s mission is to advance the quality of healthcare for patients in Georgia communities through the profession of pharmacy.
Media Contact:
Michelle Turkington
Georgia Pharmacy Association
Director of Marketing and Communications
6065 Barfield Road NE, Suite 100
Sandy Springs, Georgia 30328
mturkington@gpha.org
678-640-4313
Tell us what you want! CPE that is
You can help the GPhA CPE advisory committee deliver top-quality education content for 2021. Please take a few minutes to complete our annual needs assessment to tell us what topics interest you. CLICK HERE to get started!
GPhA Mourns the Passing of Jim Bartling
Georgia lost a pillar of the pharmacy community. It is with the deepest regret that we inform you of the passing of GPhA past President Jim Bartling, PharmD, RPh, GCADC-III, on September 24, 2020.
Jim served as president of the Georgia Pharmacy Association for 1992-93 and served on the Board of Directors of the Georgia Pharmacy Foundation — chairing the foundation until his passing. Jim also co-owned the Medical Arts Pharmacy in Conyers and worked occasionally as a relief pharmacist in the community. In 2009, he was awarded the Bowl of Hygeia, one of the most prestigious recognitions of pharmacist leaders in the state.
Jim’s passion was supporting the recovery of pharmacists, student pharmacists, and pharmacy technicians with substance use disorders. A certified addiction counselor, he was instrumental in the formation of the PharmAssist Program, one of the nation’s first state pharmacy recovery networks (PRNs) – and served as the program’s statewide intervention coordinator. For over 20 years, Dr. Bartling has headed up a private addiction counseling practice that provides outpatient treatment and aftercare monitoring for fellow pharmacists, student pharmacists, and pharmacy technicians.
Jim Bartling spent most of his professional career on the faculty of Mercer University College of Pharmacy, where he earlier earned both his bachelor of science and PharmD. He practiced at Doctors Memorial Hospital in Atlanta before joining what was then the Mercer University Southern School of Pharmacy as director of admissions, job placement, and continuing education; he was later named associate dean for student affairs and admissions, the position from which he retired in 2016. His teaching load included coordinating two elective courses – Drug Misadventures and Substance Abuse.
And even after his retirement, he remained a familiar face on the Mercer campus. Jim’s engagement in pharmacy, in healthcare, and in his community are far too numerous to list. His impact and legacy on the profession and on the Georgia Pharmacy Association and the Georgia Pharmacy Foundation will be felt forever.
You are invited to share your thoughts below with the other Georgia Pharmacy Association members.
U.S. Dept. of Health and Human Services (HHS) authorizes pharmacists to administer all ACIP recommended vaccines to children between the ages of 3 and 18 during the COVID emergency.
Today, August 18, HHS amended its declaration under the Public Readiness and Emergency Preparedness Act to authorize:
“State-licensed pharmacists to order and administer, and pharmacy interns (who are licensed or registered by their state board of pharmacy and acting under the supervision of a state-licensed pharmacist) to administer, any vaccine that the Advisory Committee on Immunization Practices (ACIP) recommends to persons ages three through 18 according to ACIP’s standard immunization schedule (ACIP-recommended vaccines).”
You can read the amendment here: https://www.hhs.gov/sites/default/files/third-amendment-declaration.pdf
This is exciting news as the federal government is recognizing the value of pharmacists and acting on it and GPhA applauds Secretary Azar for this action and is continuing to advocate for emergency action on the state level, particularly with regard to ACIP recommended vaccines as well as forthcoming COVID vaccines for children and adults. Below are the highlights of today’s amended declaration.
Preemption
- Preemption of state law for children between 3 and 18: Georgia law, which does not currently allow pharmacists to order and administer vaccines to children 3 through 12 without a prescription and 13 through 18 only in limited circumstances, is preempted under this declaration as to children between 3 and 18. As such there are no vaccine protocol requirements for children between 3 and 18 and pharmacists are authorized to administer any vaccine that ACIP recommends.
- Vaccine protocol requirements required for 18 and above remain in effect: The declaration does not preempt Georgia law with regard to administering a vaccine without a prescription to those older than 18. Remember, under Georgia law, pharmacists can administer vaccines without a specific prescription for influenza, pneumococcal disease, shingles, or meningitis pursuant to a vaccine protocol agreement and other requirements set forth in O.C.G.A. 43-34-26.1.
Requirements
The authorization applies to Georgia licensed pharmacists and their interns acting under their supervision (intern must be licensed or registered by the Georgia Board of Pharmacy) pursuant to the following requirements:
- The vaccine must be FDA-authorized or FDA-licensed.
- The vaccination must be ordered and administered according to ACIP’s standard immunization schedule.
- The licensed pharmacist must complete a practical training program of at least 20 hours that is approved by the Accreditation Council for Pharmacy Education (ACPE). This training program must include hands-on injection technique, clinical evaluation of indications and contraindications of vaccines, and the recognition and treatment of emergency reactions to vaccines.
- The licensed or registered pharmacy intern must complete a practical training program that is approved by the ACPE. This training program must include hands-on injection technique, clinical evaluation of indications and contraindications of vaccines, and the recognition and treatment of emergency reactions to vaccines.
- The licensed pharmacist and licensed or registered pharmacy intern must have a current certificate in basic cardiopulmonary resuscitation.
- The licensed pharmacist must complete a minimum of two hours of ACPE-approved, immunization-related continuing pharmacy education during each state licensing period.
- The licensed pharmacist must comply with recordkeeping and reporting requirements of the jurisdiction in which he or she administers vaccines, including informing the patient’s primary-care provider when available, submitting the required immunization information to the State or local immunization information system (vaccine registry), complying with requirements with respect to reporting adverse events, and complying with requirements whereby the person administering a vaccine must review the vaccine registry or other vaccination records prior to administering a vaccine.
- The licensed pharmacist must inform his or her childhood-vaccination patients and the adult caregivers accompanying the children of the importance of a well-child visit with a pediatrician or other licensed primary-care provider and refer patients as appropriate.
Note 1: Some of the above requirements are consistent with those under Georgia law and some are more stringent. By way of example in Georgia pharmacists have to report to GRITS but don’t have to check prior to administering. The above is more stringent, providing pharmacists must “review vaccine registry or other vaccination records prior to administering a vaccine.” In other words, don’t assume because you have met the requirements for a vaccine protocol agreement in Georgia you are good to go.
Note: 2: With regard to record-keeping and reporting, the authorization references complying with “record-keeping and reporting requirements of the jurisdiction.” Pharmacists would do well to comply with record-keeping and reporting requirements set forth in O.C.G.A. 43-34-26.1 in addition to any more stringent requirements set forth above.
2020 Legislative Session Wrap-up
Greg Reybold, General Counsel & VP of Public Policy, Georgia Pharmacy Association
Georgia’s 2020 session was, to put it mildly, a unique one. To begin with, GPhA’s initiatives were far reaching, and, following the success of 2019, fiercely contested by lobbyists for PBMs, insurance companies, as well as Medicaid care management organizations.
That combined with legislative session being suspended for well over two months following cross-over day, the budget needing to be approved with significant cuts, and a limited number of bills being taken up by the general assembly for the final 11 days of legislative session, made for head winds the likes of which we have never seen.
Nonetheless, GPhA, and its members, remained focused on its legislative initiatives. That focus resulted in, despite the adversity, one of GPhA’s most successful legislative sessions in history with the passage of four pieces of priority legislation as well as the engagement of an important study.
These bills now go to the Governor, with the hopes that they receive his favorable consideration and are signed into law.
As you read through the details of GPhA priority legislation please keep in mind that GPhA members were an integral part of the successes of this landmark legislative session. Time and again you answered the call. You were, however, not alone. Please join us in extending appreciation to the National MS Society and their Senior Manager of Advocacy, Heather Breeden; the Rx in Reach Coalition and Advocates for Responsible Care and their Executive Director, Dorothy Leone-Glasser; the Georgia Society of Clinical Oncology; and the Medical Association of Georgia.
We owe a special debt of gratitude to Representatives Knight, Cooper, Hatchett, England, Smith, Newton, Burns, Holmes, Stephens, and House Speaker Ralston. On the senate side we owe a special debt of gratitude to Senators Burke, Watson, Jones, Hufstetler, Tillery, Mullis, and Lt. Governor Geoff Duncan.
Should these bills become law, there will be more detail coming via the Georgia law update which will address the intricacies of the legislation including implementation dates, scope, and enforcement.
Finally, the body of work from the General Assembly and GPhA over the last several years represents what we believe to be amongst the most comprehensive and material legislation in the nation. Though it is not a cure for all problems with PBMs, and PBMs will continue to assert alleged grounds for not adhering, the legislation is a testament to the regard with which pharmacists and their patients are held both by GPhA and Georgia’s General Assembly. Of thousands of issues the General Assembly can tackle, they continue to do everything they can to fight for you and your patients. Please remember that, and, thank your representative and senator the next time you seem them.
PBM Reform
HB 946 (Rep. Knight) & SB 313 (Sen. Burke) represent a comprehensive rewrite of Georgia’s PBM code section (Chapter 64 of Title 33) that include some belt and suspenders changes to strengthen oversight and enforcement as well as innovative first in the nation changes designed to increase transparency, level the playing field and protect patients. Set forth below are highlights of the changes this bill contemplates.
Licensing and oversight
- Removes barriers on Commissioner enforcement.
- Provides the Commissioner of Insurance (“Commissioner”) the power to suspend PBM licenses for violation of the law.
- Gives the Commissioner the authority to conduct financial examinations and compliance audits; issue cease and desist orders; order reimbursement to a pharmacy or insured when a monetary loss has been incurred as a result of a PBM violation; and order payment of a fine up to $1,000 to go to a pharmacy or insured.
- Requires a PBM to make its records available to the Commissioner.
- Empowers the Commissioner to conduct audits following a violation to identify any similar violations.
- Requires PBMs to file MAC methodologies with the Commissioner’s office to enable Commissioner to investigate MAC complaints.
- Increases monetary fines from $1,000 to $2,000 per violation and up to $10,000 per violation when a PBM knew or should have reasonably known it was in violation.
- Increases licensing fee from $500 to $2,000 and renewal fee from $400 to $1,000.
Transparency
- Prohibits PBMs from imposing point of sale or retroactive fees
- Requires reporting of drugs paid 10% above and 10% below NADAC every four months and making the reports available to the public via a website.
- Prohibits differential treatment of 340b pharmacies.
- Prohibits PBMs from basing reimbursement of a drug on patient scores or outcomes.
- Requires PBMs offer plans the ability to receive 100% of all rebates (broad definition of rebate).
- Requires PBMs offer plans non-spread pricing options.
- Prohibits PBMs from engaging in the practice of spread pricing in state, county, and municipality plans.
Patient Protections
- Requires PBM contracted or employed physicians who make prior authorization and step therapy decisions in connection with appeals to practice in same specialty area in which they are advising.
- Strengthens steering prohibition to prohibit penalizing patients and plans when a patient uses a non-PBM affiliated in network pharmacy of their choice and prohibits PBM to PBM cross referral arrangements.
- Prohibits PBMs from deriving revenue from a pharmacy or patient.
- Prohibits PBMs from withholding coverage or requiring a prior authorization for a lower cost therapeutically equivalent drug.
- Prohibits PBMs from removing a drug from a formulary for the purpose of incentivizing an insured to seek coverage elsewhere.
- Requires PBMs to apply accepted copay assistance where there is only a brand name drug available to a patient’s copay and deductible.
- Strengthens ability of retail pharmacies to provide delivery services to their patients.
PBM Surcharge
- Imposes a first in the nation surcharge on PBMs and insurer clients on all claims administered when the PBM engages in the practices of steering or imposing retroactive fees. This surcharge is imposed for the purpose of encouraging payors to use PBMs that are not engaging in these prohibited practices.
Applicability to Medicaid managed care
- Removes Medicaid managed care company exemptions so that all prohibitions and patient protections apply in the Medicaid managed care market.
Pharmacy Steering & Audits
Building off of HB 233 which was passed in 2019, HB 918 (Rep. Sharon Cooper) strengthens anti-steering provisions which prohibit pharmacies affiliated with PBMs from filling and billing for prescriptions illegally referred and also remove certain exemptions and loopholes. In addition, this bill makes significant improvements to the Pharmacy Audit Bill of Rights.
Steering
- Removes language relied upon by DCH and CVS as justification for continued steering. This language also exempted PBM affiliated pharmacies from other pharmacy requirements.
- Strengthens anti-steering law by prohibiting steering via monetary penalties including withholding coverage/requiring patients to pay full cost of drug & prohibiting PBM to PBM pharmacy referral arrangements.
- Applies anti-steering protections to limited distribution drugs not commonly carried at pharmacies or oncology clinics.
- Applies anti-steering law to Medicaid managed care companies.
Pharmacy Audit Bill of Rights
- Applies protections to desk audits as well as on-site audits.
- Limits audits to no more than 100 prescriptions and no more than 200 prescriptions in a year (refills count as 1 prescription).
- Broadens “clerical error” to include omission errors.
- Expands period to correct a clerical error from 20 to 60 days.
- Expedites period preliminary audit report must be delivered from 120 day up to 30 days.
- Prohibits a PBM from imposing a penalty or fee in connection with an audit.
- Prohibits recoupment from a pharmacy except in cases of fraud; overpayment (limited to amount over paid); and misfill. Provides that when a patient receives the correct drug in the correct dosage and quantity pursuant to a prescription drug order than no misfill shall be found to have occurred.
- Provides that a PBM shall not audit a pharmacy more than once every six months.
- Increases Commissioner of Insurance oversight and ability to impose fines and restitution.
- * Medicaid fee for service audits and recoupments are still subject to O.C.G.A. 49-4-151.1
State Oversight
HB 991 (Rep. Hatchett) creates the Healthcare Transparency and Accountability Act which seeks to shine a light on the practices of state healthcare plans via the creation of an oversight committee; ensuring the committee has broad access to plan records; and requiring certain mandatory reporting.
- Creates oversight committee to oversee state healthcare plans and is comprised of a physician, a pharmacist, a consumer member, and 6 members of the general assembly.
- Gives committees the power to:
- Request and review records of state healthcare plan contractors and subcontractors (including PBMs); prepare public reports using aggregated data.
- Submit written questions to departments, agencies, and contractors.
- Make recommendations to departments and agencies.
- Retain third-party consultants including attorneys and actuaries.
- Request an audit of a state healthcare plan contractor or subcontractor (including PBMs) from the Department of Audits and Accounts.
- Requires contractors and subcontractors to make all books, documents, and records available to the Committee and the Department of Audits and Accounts.
- Requires a contractor to file an annual report to Committee which shall be available to the public including but not limited to amount paid by the state, MLRs, and dividends paid to shareholders or affiliates.
- Requires annual PBM report to Committee which shall be available to the public detailing, amongst other things:
- Aggregate rebates and fees collected and the amount retained.
- Aggregate pharmacy claims data.
- Aggregate amount paid to affiliate pharmacies.
- Names of 25 prescription drugs which were subject to most prior authorizations.
- Provides that an amount paid to a contractor and subcontractor is subject to disclosure to the public and is not confidential or a trade secret.
- Provides the Commissioner of Insurance with oversight and the ability to institute fines.
Medicaid Carveout Study
HB 947 (Rep. Knight) looked to, amongst other things, require DCH to engage a Medicaid actuary to conduct a study on the potential savings from carving out Rx benefits from the Medicaid managed care program and putting those benefits back into fee for service. Rep. Knight and GPhA agreed to press pause on the legislation after the Department went ahead and tasked its Medicaid actuary (the same company that conducted the West Virginia carve out study) with conducting the requested study. The study is scheduled to be completed by December of 2020. Note the midyear budget via HB 792 contained $175,000 for DCH to conduct the actuarial study.
Other legislation that passed and makes its way to the Governor’s office
- HB 759: Introduced by Rep. Parrish this bill updates the drug code.
- HB 791: introduced by Rep. Stephens this legislation authorizes pharmacist conversion of maintenance meds from 30 days to 90 days. Also included requirements for insurers to cover medications dispensed during declared emergencies which also passed via SB 391 (Sen. Kirkpatrick).
- HB 888: Introduced by Rep. Hawkins, this legislation looks to protect GA patients from surprise billing practices.
- SB 372: Introduced by Senator Tillery, this bill, amongst other things, increases the availability of naloxone to first responders via distributors.
- SB 482: Introduced by Senator Burke, this legislation creates the Georgia All Payer Claims database which will require insurers to file payment data to the state. The purpose of this legislation is to allow data driven decisions for the purpose of increasing access and quality of care while reducing costs.
- SB 359: Senator Hufstetler’s bill was substituted on the House side by Rep. Kelly to provide for protections for providers and other business owners from COVID-19 liability claims. This bill seeks to provide protection from claims of negligence but preserves claims for gross negligence and intentional torts.
2020 Legislative Update: Week 9
Week 9 of Georgia’s legislative session was both busy and filled with uncertainty as the General Assembly looked to navigate not only crossover day but also how to proceed in light of CoviD-19. Crossover day is the last day a bill may crossover from its originating chamber and so if a bill does not crossover it is no longer viable that session though its contents may be subsequently added to a viable bill. Crossover day was March 12 and the General Assembly worked deep into the night to get bills out of their originating chambers.
For GPhA, several key bills had already crossed over which made crossover day somewhat less stressful. Those bills are:
- SB 313 (Sen. Burke’s PBM bill)
- HB 918 (Rep. Cooper’s update to the Pharmacy Anti-Steering Act and Pharmacy Audit Bill of Rights)
- HB 946 (Rep. Knight’s PBM bill & companion to SB 313)
- HB 947 (Rep. Knight’s bill seeking a study of the fiscal impact of carving Rx benefits from Medicaid managed care & mandating a carve out if the study reflects potential annual savings of $20 million or more)
- HB 991 (Rep. Hatchett’s legislation creating the Healthcare Transparency and Accountability Oversight Committee)
In addition, several other bills GPhA is monitoring made crossover while others did not. Set forth below is a breakdown of those bills.
Last week the General Assembly made the decision to suspend the legislative session until further notice due to CoviD-19 concerns. The session will reconvene, and the hope is that when it does the body will continue to work on moving legislation forward in the remaining days of the session. The General Assembly also passed the midyear budget via HB 792 which, amongst other things, contained $175,000 for DCH to conduct an actuarial study on the fiscal impact of carving Rx benefits from Medicaid managed care. In a year with much belt tightening and several distracting factors, getting this money appropriated is a significant step forward for assessing the potential savings the state may achieve via a Medicaid managed care Rx carve out and for transparency.
GPhA monitored bills that made crossover
- HB 759 (drug update)
- HB 791 (authorizing conversion of maintenance meds from 30 days to 90 days)
- HB 847 (hemp farming)
- HB 888 (surprise billing)
- SB 28 (now dealing with reasonableness of copays)
- SB 272 (OTC cough syrup restrictions)
- SB 311 (restricting remuneration in connection with referral to/from substance abuse providers)
- SB 532 (online provider directories)
- SB 359 (surprise billing)
- SB 372 (amongst other things, increasing availability of naloxone via distributors)
- SB 391 (requiring coverage for medications dispensed during declared emergencies)
GPhA monitored bills that did not make crossover
- HB 813 (Medicaid expansion)
- HB 864 (excise tax on vape devices and products)
- HB 952 (restricting corporate practice of pharmacy)
- HB 961 (transparency for prescription drugs which state expends significant funds)
- HB 1027 (Dealing with PBM treatment of rebates)
- HB 1105 (patient protection through health information)
- HB 1128 (PBM reform – introduced the week of crossover)
- SB 376 (insulin costs)
- SB 415 (tort reform)
- SB 427 (Rx carve out from Medicaid managed care – companion to HB 947 which did make cross over)
- SB 433 (drug pricing transparency)
- SB 481 (exempting low THC and marijuana products approved by FDA from certain definitions)
AIP Spring Meeting cancelled
For obvious reasons, GPhA’s Academy of Independent Pharmacists has cancelled its scheduled Spring Meeting, which was going to be held April 19. If the meeting is going to be rescheduled, we’ll be in touch with members. For now, stay healthy and help your patients do the same.
Legislative Update: Session suspended
The Georgia General Assembly has suspended its 2020 session in the wake of the coronavirus.
“The House and Senate will be gaveled in Friday morning and then reconvene for the 30th legislative day “at a future date and time to be set” by the lieutenant governor and speaker of the House.”
2020 Legislative Update: Week 8
Week 8 (March 2-5 and legislative days 22-25) saw forward progress on several pieces of pharmacy related legislation.
HB 918; HB 946; and HB 947
The House passed all three of these bills this week almost unanimously with HB 946 receiving one no vote. They now make their way over to the senate for consideration. Please take the time to thank Reps. Knight and Cooper for their efforts in championing these pro-patient and pro-pharmacy pieces of legislation as well as your representative for their favorable vote.
SB 313
Senator Burke’s PBM bill passed out of the Senate unanimously this week as well. Their was an amendment on the floor which removed the requirement that PBMs pass along 100% of their rebates to plans and the prohibition on spread pricing practices. In place of those requirements, PBMs will be required to offer plans non-spread and 100% pass through options and will be required to report rebates and spread retained by PBMs. GPhA was involved in the crafting of this amendment. Please take the time to reach out to Senator Burke and thank him for his support and leadership as well as Senator Burt Jones who chaired the Insurance and Labor Committee and who was instrumental in the Senate’s passage of this legislation. Please also thank your Senator for their vote in favor of this bill.
Legislation introduced in week 8
SB 481
Introduced by Senator Brass, this legislation removes products approved by the FDA from the definitions of low THC oil, marijuana, and tetrahydrocannabinols.
Other previously introduced legislation
HB 888, Rep. Hawkins’ surprising billing legislation, passed the House and makes it way to the Senate.
HB 991, Rep. Hatchett’s bill creating the Healthcare Transparency and Accountability Oversight Committee, was favorably reported by the House Special Committee on Access to Quality Healthcare. GPhA testified in favor of this legislation and this legislation remains a GPhA priority to support.
SB 311, Sen. Kirkpatrick’s bill prohibiting kickbacks and patient brokering in connection with substance abuse providers was favorably reported by Senate HHS via substitute.
SB 391, Sen. Kirkpatrick’s legislation requiring insurers to cover early fills of medication during declared emergencies was favorably reported by the Senate HHS Committee.
◀ Newer Posts Older Posts ▶